![]() NEET 2018
NEET 2018
|
Section |
Questions |
Marks |
|
Chemistry |
45 Questions (1 – 45) |
180 |
|
Biology |
90 Questions (46 – 135) |
360 |
|
Physics |
45 Questions (136 – 180) |
180 |
![]() Q. 1 Which of the following statements is “not” true for the halogens?
Q. 1 Which of the following statements is “not” true for the halogens?
A. All form monobasic oxyacids
B. Chlorine has the highest electron gain enthalpy
C. All but fluorine show positive oxidation states
D. All are oxidizing agents
![]() Q. 2 The correct order of atomic radii in group 13 elements are:
Q. 2 The correct order of atomic radii in group 13 elements are:
A. B < Al < In < Ga < Tl
B. B < Ga < Al < In < Tl
C. B < Ga < Al < Tl < In
D. B < Al < Ga < In < Tl
![]() Q. 3 In the structure of CIF₃ , the number of lone pairs of electrons on the central atom “Cl” is:
Q. 3 In the structure of CIF₃ , the number of lone pairs of electrons on the central atom “Cl” is:
A. One
B. Three
C. Four
D. Two
![]() Q. 4 The correct order of the N-compounds in its decreasing order of oxidation states is:
Q. 4 The correct order of the N-compounds in its decreasing order of oxidation states is:
A. HNO₃, NO, N₂, NH₄Cl
B. NH₄Cl, N₂, NO, HNO₃
C. HNO₃, NH₄Cl, NO, N₂
D. HNO₃, NO, NH₄Cl, N₂
![]() Q. 5 Which one of the following elements is unable to form MF₆⁻³ ion:
Q. 5 Which one of the following elements is unable to form MF₆⁻³ ion:
A. Ga
B. In
C. B
D. Al
![]() Q. 6 Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
Q. 6 Considering Ellingham diagram, which of the following metals can be used to reduce alumina?
A. Fe
B. Cu
C. Mg
D. Zn
![]() Q. 7 The compound A on treatment with Na gives B, and with PCl₅ gives C. B and C react together to give diethyl ether. A, B and C are in the order
Q. 7 The compound A on treatment with Na gives B, and with PCl₅ gives C. B and C react together to give diethyl ether. A, B and C are in the order
A. C₂H₅OH, C₂H6, C₂H₅Cl
B. C₂H₅OH, C₂H₅ONa, C₂H₅Cl
C. C₂H₅Cl, C₂H6, C₂H₅OH
D. C₂H₅OH, C₂H₅Cl, C₂H₅ONa
![]() Q. 8 Hydrocarbon (A) reacts with bromine by substitution to form an alkyl bromide which by Wurtz reaction is converted to gaseous hydrocarbon containing less than four carbon atoms (A) is :
Q. 8 Hydrocarbon (A) reacts with bromine by substitution to form an alkyl bromide which by Wurtz reaction is converted to gaseous hydrocarbon containing less than four carbon atoms (A) is :
A. CH = CH
B. CH₄
C. CH₃ – CH₃
D. CH₂ = CH₂
![]() Q. 9 The compound C₇H₈ undergoes the following reactions given in the figure. The product ‘C’ is:
Q. 9 The compound C₇H₈ undergoes the following reactions given in the figure. The product ‘C’ is:
A. m-bromotoluene
B. p-bromotoluene
C. 3-bromo-2,4,6-trichlorotoluene
D. o-bromotoluene
![]() Q. 10 Which oxide of nitrogen is not a common pollutant introduced into the atmosphere both due to natural and human activity?
Q. 10 Which oxide of nitrogen is not a common pollutant introduced into the atmosphere both due to natural and human activity?
A. N₂O₅
B. NO
C. N₂O
D. NO₂
![]() Q. 11 Following solutions were prepared by mixing different volumes of NaOH and HCl of different concentrations:
Q. 11 Following solutions were prepared by mixing different volumes of NaOH and HCl of different concentrations:
a. 60mL M/10 HCl + 40mL M/10 NaOH
b. 55mL M/10 HCl + 45mL M/10 NaOH
c. 75mL M/5 HCl + 25mL M/5 NaOH
d. 100mL M/10 HCl + 100mL M/10 NaOH
pH of which one of them will be equal to 1?
A. b
B. c
C. d
D. a
![]() Q. 12 On which of the following properties does the coagulating power of an ion depend?
Q. 12 On which of the following properties does the coagulating power of an ion depend?
A. The magnitude of the charge on the ion alone
B. The sign of charge on the ion alone
C. Both magnitude and sign of the charge on the ion
D. Size of the ion alone
![]() Q. 13 The solubility of BaSO₄ in water is 2.42 x 10⁻³ gL⁻¹ at 298K.The value of its solubility product (Ksp) will be (Given molar mass of BaSO₄=233g/mol⁻¹)
Q. 13 The solubility of BaSO₄ in water is 2.42 x 10⁻³ gL⁻¹ at 298K.The value of its solubility product (Ksp) will be (Given molar mass of BaSO₄=233g/mol⁻¹)
A. 1.08 x 10⁻¹⁰ mol²/L⁻²
B. 1.08 x 10⁻⁸ mol²/L⁻²
C. 1.08 x 10⁻¹⁴ mol²/L⁻²
D. 1.08 x 10⁻¹² mol²/L⁻²
![]() Q. 14 Given van der Waals constant for NH₃, H₂, O₂ and CO₂ are respectively 4.17, 0.244, 1.36 and 3.59, which one of the following gases is most easily liquefied?
Q. 14 Given van der Waals constant for NH₃, H₂, O₂ and CO₂ are respectively 4.17, 0.244, 1.36 and 3.59, which one of the following gases is most easily liquefied?
A. NH₃
B. CO₂
C. O₂
D. H₂
![]() Q. 15 Match the metal ions given in column I with the spin magnetic moments of the ions given in column II (Given in figure (1)) and assign the correct code:
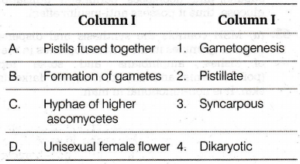
Q. 15 Match the metal ions given in column I with the spin magnetic moments of the ions given in column II (Given in figure (1)) and assign the correct code:
A. a(iv), b(v), c(ii), d(i)
B. a(iii), b(v), c(i), d(ii)
C. a(iv), b(i), c(ii), d(iii)
D. a(i), b(ii), c(iii), d(iv)
![]() Q. 16 Iron carbonyl, Fe(CO)₅ is:
Q. 16 Iron carbonyl, Fe(CO)₅ is:
A. tetranuclear
B. dinuclear
C. trinuclear
D. mononuclear
![]() Q. 17 The geometry and magnetic behaviour of the complex [Ni(CO)₄] are:
Q. 17 The geometry and magnetic behaviour of the complex [Ni(CO)₄] are:
A. square planar geometry and diamagnetic
B. tetrahedral geometry and paramagnetic
C. square planar geometry and paramagnetic
D. tetrahedral geometry and diamagnetic
![]() Q. 18 Which one of the following ions exhibits d-d transition and paramagnetism as well?
Q. 18 Which one of the following ions exhibits d-d transition and paramagnetism as well?
A. CrO₄⁻²
B. MnO₄⁻²
C. MnO₄⁻
D. Cr₂O₇⁻²
![]() Q. 19 The type of isomerism shown by the complex [CoCl₂(en)₂] is:
Q. 19 The type of isomerism shown by the complex [CoCl₂(en)₂] is:
A. geometrical isomerism
B. linkage isomerism
C. ionisation isomerism
D. coordination isomerism
![]() Q. 20 Identify the major products P, Q and R (Among options (1), (2), (3), (4)) in the following sequence of reaction given in the figure:
Q. 20 Identify the major products P, Q and R (Among options (1), (2), (3), (4)) in the following sequence of reaction given in the figure:
A. 1
B. 2
C. 3
D. 4
![]() Q. 21 Which of the following compounds can form a zwitterion?
Q. 21 Which of the following compounds can form a zwitterion?
A. Aniline
B. Glycine
C. Benzoic acid
D. Acetanilide
![]() Q. 22 Which of the following molecules represent the order of hybridisation sp², sp², sp, sp from left to right atoms?
Q. 22 Which of the following molecules represent the order of hybridisation sp², sp², sp, sp from left to right atoms?
A. HC ≡ C – C ≡ CH
B. CH₃ – CH = CH – CH₃
C. CH₂ = CH – CH = CH₂
D. CH₂ = CH – C = CH
![]() Q. 23 Which of the following carbocations is expected to be most stable (Among options (1), (2), (3), (4)):
Q. 23 Which of the following carbocations is expected to be most stable (Among options (1), (2), (3), (4)):
A. 1
B. 2
C. 3
D. 4
![]() Q. 24 Which of the following is correct with respect to -I effect of the substituents?(R = alkyl)
Q. 24 Which of the following is correct with respect to -I effect of the substituents?(R = alkyl)
A. -NH₂ < -OR < -F
B. -NR₂ > -OR > -F
C. -NH₂ > -OR > -F
D. -NR₂ < -OR < -F
![]() Q. 25 Magnesium reacts with an element (X) to form an ionic compound. If the ground state electronic configuration of (X) is 1s² 2s² 2p³, the simplest formula for this compound is:
Q. 25 Magnesium reacts with an element (X) to form an ionic compound. If the ground state electronic configuration of (X) is 1s² 2s² 2p³, the simplest formula for this compound is:
A. Mg₂X₃
B. Mg₃X₂
C. Mg₂X
D. MgX₂
![]() Q. 26 Iron exhibits bcc structure at room temperature. Above 900°C, it transforms to fcc structure. The ratio of density of iron at room temperature to that at 900°C (assuming molar mass and atomic radii of iron remains constant with temperature) is:
Q. 26 Iron exhibits bcc structure at room temperature. Above 900°C, it transforms to fcc structure. The ratio of density of iron at room temperature to that at 900°C (assuming molar mass and atomic radii of iron remains constant with temperature) is:
A. √3/√2
B. 1/2
C. 3√3/4√2
D. 4√3/3√2
![]() Q. 27 Which of the following statement is wrong (Among (1), (2), (3), (4))?
Q. 27 Which of the following statement is wrong (Among (1), (2), (3), (4))?
A. 1
B. 2
C. 3
D. 4
![]() Q. 28 Consider the following species:
Q. 28 Consider the following species:
CN⁺, CN⁻, NO and CN
Which one of these will have the highest bond order?
A. NO
B. CN
C. CN⁺
D. CN⁻
![]() Q. 29 In the reaction given in the figure, the electrolyte involved is:
Q. 29 In the reaction given in the figure, the electrolyte involved is:
A. dichloromethyl cation (CHCl₂)
B. dichlorocarbene (:CCl₂)
C. dichloromethyl anion (CHCl₂)
D. formyl cation (CHO)
![]() Q. 30 Carboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their:
Q. 30 Carboxylic acids have higher boiling points than aldehydes, ketones and even alcohols of comparable molecular mass. It is due to their:
A. formation of intramolecular H-bonding
B. formation of intermolecular H-bonding
C. more extensive association of carboxylic acid via van der Waals force of attraction
D. formation of carboxylate ion
![]() Q. 31 Compound A, C₈H₁₀O is found to react with NaOI (produced by reacting Y with NaOH) and yields a yellow precipitate with characteristic smell.
Q. 31 Compound A, C₈H₁₀O is found to react with NaOI (produced by reacting Y with NaOH) and yields a yellow precipitate with characteristic smell.
A and Y are respectively (Among (1), (2), (3), (4)):
A. 1
B. 2
C. 3
D. 4
![]() Q. 32 The correct difference between first and second order reactions is that:
Q. 32 The correct difference between first and second order reactions is that:
A. The rate of first order reaction does not depend on the reactant concentrations; the rate of a second order reaction does depend on reactant concentrations.
B. The rate of first order reaction does depend on the reactant concentrations; the rate of a second order reaction does not depend on the reactant concentrations.
C. a first order reaction can be catalyzed; a second order reaction cannot be catalyzed.
D. the half time of a first order reaction does not depend on the [A]0; the half life of a second order reaction does not depend on the [A]0
![]() Q. 33 Among the CaH₂, BeH₂, BaH₂, the order of the ionic character is:
Q. 33 Among the CaH₂, BeH₂, BaH₂, the order of the ionic character is:
A. BeH₂ < CaH₂ < BaH₂
B. BaH₂ < BeH₂ < CaH₂
C. BeH₂ < BaH₂ < CaH₂
D. CaH₂ < BeH₂ < BaH₂
![]() Q. 34 Consider the change in oxidation state of Bromine corresponding to different emf values as shown in the diagram.
Q. 34 Consider the change in oxidation state of Bromine corresponding to different emf values as shown in the diagram.
Then the species undergoing disproportionation is:
A. BrO₃⁻
B. HBrO
C. Br₂
D. BrO₄⁻
![]() Q. 35 In which case is the number of molecules of water maximum?
Q. 35 In which case is the number of molecules of water maximum?
A. 18 mL of water
B. 10⁻³ mol of water
C. 0.00224L of water vapours at 1 atm and 273 K
D. 0.18 g of water
![]() Q. 36 Regarding cross-linked or network polymers, which of the following statements is incorrect?
Q. 36 Regarding cross-linked or network polymers, which of the following statements is incorrect?
A. They contains covalent bonds between various linear polymer chains.
B. They contains strong covalent bonds in their polymer chains
C. Examples are bakelite and melamine
D. They are formed from bi and tri functional monomers.
![]() Q. 37 Nitration of aniline in strong acidic medium also gives m-nitroaniline because:
Q. 37 Nitration of aniline in strong acidic medium also gives m-nitroaniline because:
A. In spite of substitutions nitro group always goes to only m-position
B. In acidic (strong) medium aniline is present as anilinium ion
C. In absence of substituents nitro groups always goes to m-position
D. In electrophilic substitution reactions amino group is meta directive
![]() Q. 38 Which of the following oxides is most acidic in nature?
Q. 38 Which of the following oxides is most acidic in nature?
A. MgO
B. CaO
C. BaO
D. BeO
![]() Q. 39 The difference between amylose and amylose and amylopectin is:
Q. 39 The difference between amylose and amylose and amylopectin is:
A. Amylopectin have 1 → 4 α-linkage and 1 → 6 α-linkage
B. Amylose is made up of glucose and galactose
C. Amylopectin have 1 → 4 α-linkage and 1→ 6 β-linkage
D. Amylose have 1 → 4 α-linkage and 1 → 6 β-linkage
![]() Q. 40 A mixture of 2:3 g formic acid and 4.5 g oxalic acid is treated with conc. H₂SO₄. The evolved gaseous mixture is passed through KOH pallets. Weight (in g) of the remaining product at STP will be:
Q. 40 A mixture of 2:3 g formic acid and 4.5 g oxalic acid is treated with conc. H₂SO₄. The evolved gaseous mixture is passed through KOH pallets. Weight (in g) of the remaining product at STP will be:
A. 1.4
B. 4.4
C. 2.8
D. 3.0
![]() Q. 41 For redox reaction given in the figure, what is the correct coefficients of the reactants for the balanced equation are:
Q. 41 For redox reaction given in the figure, what is the correct coefficients of the reactants for the balanced equation are:
A. 1
B. 2
C. 3
D. 4
![]() Q. 42 The correction factor ‘a’ to the ideal gas equation corresponds to:
Q. 42 The correction factor ‘a’ to the ideal gas equation corresponds to:
A. density of the gas molecules
B. forces of attraction between the gas molecules
C. electric field present between the gas molecules
D. volume of the gas molecules
![]() Q. 43 Which one of the following conditions will favour maximum formation of the product in the reaction:
Q. 43 Which one of the following conditions will favour maximum formation of the product in the reaction:
A₂(g) + B₂(G) ⇔ X₂(g) ΔrH = – X kJ?
A. low temperature and high pressure
B. high temperature and low temperature
C. high temperature and high pressure
D. low temperature and low pressure
![]() Q. 44 The bond dissociation energies of X₂, Y₂ and XY of in the ratio of 1 : 0.5 : 1. ΔH for the formation of XY is -200 KJ/mol⁻¹.The bond dissociation energy of X₂ will be:
Q. 44 The bond dissociation energies of X₂, Y₂ and XY of in the ratio of 1 : 0.5 : 1. ΔH for the formation of XY is -200 KJ/mol⁻¹.The bond dissociation energy of X₂ will be:
A. 200KJ mol⁻¹
B. 400 KJ mol⁻¹
C. 800 KJ mol⁻¹
D. 100 KJ mol⁻¹
![]() Q. 45 When initial concentration of the reactant is doubled, the half life period of a zero order reaction.
Q. 45 When initial concentration of the reactant is doubled, the half life period of a zero order reaction.
A. is halved
B. remains unchanged
C. is tripled
D. is doubled
![]() Q. 46 Which of the following is an occupational respiratory disorder?
Q. 46 Which of the following is an occupational respiratory disorder?
A. anthracis
B. emphysema
C. botulism
D. silicosis
![]() Q. 47 Calcium is important in skeletal muscle contraction because it:
Q. 47 Calcium is important in skeletal muscle contraction because it:
A. binds to troponin to remove the masking of active sites on actin for myosin
B. prevents the formation of bonds between the myosin cross bridges and the actin filament
C. detaches the myosin head from the actin filament
D. activates the myosin ATPase by binding to it
![]() Q. 48 Which of the following gastric cells indirectly help in erythropoiesis?
Q. 48 Which of the following gastric cells indirectly help in erythropoiesis?
A. chief cells
B. parietal cells
C. goblet cells
D. mucous cells
![]() Q. 49 Match the items given in column I with those in column II (Given in the figure) and select the correct option:
Q. 49 Match the items given in column I with those in column II (Given in the figure) and select the correct option:
A. a(iii), b(ii), c(i)
B. a(ii), b(iii), c(i)
C. a(i), b(iii), c(ii)
D. a(i), b(ii), c(iii)
![]() Q. 50 Which of the following hormones can play a significant role in osteoporosis?
Q. 50 Which of the following hormones can play a significant role in osteoporosis?
A. Aldosterone and Prolactin
B. Parathyroid hormone and Prolactin
C. Estrogen and Parathyroid hormone
D. Progesterone and Aldosterone
![]() Q. 51 Which of the following is an amino acid derived hormone?
Q. 51 Which of the following is an amino acid derived hormone?
A. epinephrine
B. estriol
C. estradiol
D. ecdysone
![]() Q. 52 Which of the following structures or regions is incorrectly paired with its function?
Q. 52 Which of the following structures or regions is incorrectly paired with its function?
A. Medulla oblongata: It controls respiration and cardiovascular reflexes
B. Corpus callosum: band of fibres connecting the left and right cerebral hemispheres
C. Hypothalamus: production of releasing hormones and regulation of temperature, hunger and thirst
D. Limbic system: consists of fibre tracts that interconnect different regions of brain; controls movements.
![]() Q. 53 The transparent lens in the human eye is held in its place by
Q. 53 The transparent lens in the human eye is held in its place by
A. ligaments attached to ciliary body
B. smooth muscles attached to the ciliary body
C. smooth muscles attached to the iris
D. ligaments attached to the iris
![]() Q. 54 Among the following set of examples for divergent evolution, select the incorrect option.
Q. 54 Among the following set of examples for divergent evolution, select the incorrect option.
A. forelimbs of man, bat and cheetah
B. eye of octopus, bat and man
C. brain of bat, man and cheetah
D. heart of bat, man and cheetah
![]() Q. 55 In which disease does mosquito transmitted pathogen cause chronic inflammation of lymphatic vessels?
Q. 55 In which disease does mosquito transmitted pathogen cause chronic inflammation of lymphatic vessels?
A. Elephantiasis
B. Amoebiasis
C. Ringworm disease
D. Ascariasis
![]() Q. 56 Which of the following is not an autoimmune disease?
Q. 56 Which of the following is not an autoimmune disease?
A. Psoriasis
B. Vitiligo
C. Alzheimer’s disease
D. Rheumatoid arthritis
![]() Q. 57 Conversion of milk to curd improves its nutritional value by increasing the amount of:
Q. 57 Conversion of milk to curd improves its nutritional value by increasing the amount of:
A. Vitamin D
B. Vitamin E
C. Vitamin B12
D. Vitamin A
![]() Q. 58 Which of the following characters represent ‘inheritance of blood groups’ in human:
Q. 58 Which of the following characters represent ‘inheritance of blood groups’ in human:
a. Dominance
b. Co-dominance
c. Multiple allele
d. Incomplete dominance
e. Polygenic inheritance
A. b, c and e
B. a, c and e
C. b, d and e
D. a, b and c
![]() Q. 59 The similarity of bone structure in the forelimbs of many vertebrates is an example of:
Q. 59 The similarity of bone structure in the forelimbs of many vertebrates is an example of:
A. homology
B. adaptive radiation
C. convergent evolution
D. analogy
![]() Q. 60 Which of the following animals does not undergo metamorphosis?
Q. 60 Which of the following animals does not undergo metamorphosis?
A. earthworm
B. starfish
C. moth
D. tunicate
![]() Q. 61 Which one of these animals is not a homeotherm?
Q. 61 Which one of these animals is not a homeotherm?
A. Macropus
B. Psittacula
C. Camelus
D. Chelone
![]() Q. 62 Which of the following features is used to identify a male cockroach from a female cockroach?
Q. 62 Which of the following features is used to identify a male cockroach from a female cockroach?
A. Presence of a boat shaped sternum on the 9th abdominal segment
B. Presence of anal cerci
C. Forewings with darker tegmina
D. Presence of caudal styles
![]() Q. 63 Which of the following organisms are known as chief producers in the oceans?
Q. 63 Which of the following organisms are known as chief producers in the oceans?
A. Dinoflagellates
B. Euglenoids
C. Cyanobacteria
D. Diatoms
![]() Q. 64 Ciliates differ from all other protozoans in
Q. 64 Ciliates differ from all other protozoans in
A. using flagella for locomotion
B. having two types of nuclei
C. using pseudopodia for capturing prey
D. having a contractile vacuole for removing excess water
![]() Q. 65 Identify the vertebrate group of animals characterized by crop and gizzard in its digestive system
Q. 65 Identify the vertebrate group of animals characterized by crop and gizzard in its digestive system
A. Amphibia
B. Osteichthyes
C. Aves
D. Reptilia
![]() Q. 66 The amnion of mammalian embryo is derived from:
Q. 66 The amnion of mammalian embryo is derived from:
A. ectoderm and mesoderm
B. ectoderm and endoderm
C. mesoderm and trophoblast
D. endoderm and mesoderm
![]() Q. 67 Hormones secreted by the placenta to maintain pregnancy are:
Q. 67 Hormones secreted by the placenta to maintain pregnancy are:
A. hCG, hPL , progestogens, prolactin
B. hCG, progestogens, estrogens, glucocorticoids
C. hCG, hPL, progestogens, estrogens
D. hCG, hPL, estrogens, relaxin, oxytocin
![]() Q. 68 The contraceptive ‘SAHELI’
Q. 68 The contraceptive ‘SAHELI’
A. blocks estrogen receptors in the uterus, preventing eggs from getting implanted
B. is a post coital contraceptive
C. is an IUD
D. increases the concentration of estrogen and prevents ovulation in females.
![]() Q. 69 The difference between spermiogenesis and spermiation is:
Q. 69 The difference between spermiogenesis and spermiation is:
A. In spermatogenesis spermatids are formed, while in spermiation spermatozoa are formed
B. In spermatogenesis spermatozoa are formed, while in spermiation spermatozoa are released from sertoli cells into the cavity of seminiferous tubules
C. In spermatogenesis spermatozoa from sertoli cells are released into the cavity of seminiferous tubules, while in spermiation spermatozoa are formed
D. In spermatogenesis spermatozoa are formed while in spermiation spermatids are formed
![]() Q. 70 In a growing population of a country:
Q. 70 In a growing population of a country:
A. pre-reproductive individuals are more than the reproductive individuals
B. pre-reproductive individuals are less than the reproductive individuals
C. reproductive and pre-reproductive individuals are equal in number
D. reproductive individuals are less than the post reproductive individuals
![]() Q. 71 Match the items given in the column I with those in column II (Given in the figure) and select the correct option given below:
Q. 71 Match the items given in the column I with those in column II (Given in the figure) and select the correct option given below:
A. a(ii), b(i), c(iii), d(iv)
B. a(i), b(ii), c(iv), d(iii)
C. a(iii), b(iv), c(i), d(ii)
D. a(i), b(iii), c(iv), d(ii)
![]() Q. 72 Which part of poppy plant is used to obtain the drug ‘smack’ ?
Q. 72 Which part of poppy plant is used to obtain the drug ‘smack’ ?
A. flowers
B. leaves
C. roots
D. latex
![]() Q. 73 Which of the following population interactions widely used in medical sciences for the production of antibiotics?
Q. 73 Which of the following population interactions widely used in medical sciences for the production of antibiotics?
A. Commensalism
B. Amensalism
C. Parasitism
D. Mutualism
![]() Q. 74 All of the following are included in “ex-situ conservation” except
Q. 74 All of the following are included in “ex-situ conservation” except
A. wildlife safari parks
B. seed banks
C. botanical gardens
D. sacred groves
![]() Q. 75 Match the items given in column I with those column II (Given in the figure) and select the correct option given below:
Q. 75 Match the items given in column I with those column II (Given in the figure) and select the correct option given below:
A. a(iii), b(ii), c(iv), d(i)
B. a(iv), b(i), c(ii), d(iii)
C. a(ii), b(iii), c(i), d(iv)
D. a(i), b(ii), c(iii), d(iv)
![]() Q. 76 Match the items given in column I with those in column II and select the correct option given below:
Q. 76 Match the items given in column I with those in column II and select the correct option given below:
A. a(iv), b(v), c(ii), d(iii)
B. a(v), b(iv), c(i), d(iii)
C. a(v), b(iv), c(i), d(ii)
D. a(iv), b(i), c(ii), d(iii)
![]() Q. 77 A woman has an X-linked condition on one of her X chromosomes. This chromosome can be inherited by:
Q. 77 A woman has an X-linked condition on one of her X chromosomes. This chromosome can be inherited by:
A. only daughters
B. both sons and daughters
C. only grandchildren
D. only sons
![]() Q. 78 AGGTATCGCAT is a sequence from the coding strand of a gene. What will be the corresponding sequence of the transcribed mRNA?
Q. 78 AGGTATCGCAT is a sequence from the coding strand of a gene. What will be the corresponding sequence of the transcribed mRNA?
A. AGGUAUCGCAU
B. UCCAUAGCGUA
C. ACCUAUGCGAU
D. UGGTUTCGCAT
![]() Q. 79 Match the items given in column I with those in column II (Given in the figure) and select the correct option given below:
Q. 79 Match the items given in column I with those in column II (Given in the figure) and select the correct option given below:
A. a(iii), b(ii), c(i)
B. a(iii), b(i), c(ii)
C. a(ii), b(iii), c(i)
D. a(i), b(iii), c(ii)
![]() Q. 80 According to Hugo de Vries, the mechanism of evolution is:
Q. 80 According to Hugo de Vries, the mechanism of evolution is:
A. multiple step mutations
B. minor mutations
C. phenotypic variations
D. saltation
![]() Q. 81 All of the following are part of an operon except
Q. 81 All of the following are part of an operon except
A. an operator
B. a promoter
C. an enhancer
D. structural genes
![]() Q. 82 Which of the following events does not occur in rough endoplasmic reticulum?
Q. 82 Which of the following events does not occur in rough endoplasmic reticulum?
A. protein folding
B. phospholipid synthesis
C. cleavage of signal peptide
D. protein glycosylation
![]() Q. 83 Which of these statements is incorrect?
Q. 83 Which of these statements is incorrect?
A. enzymes of TCA cycle are present in mitochondrial matrix
B. oxidative phosphorylation takes place in outer mitochondrial membrane
C. glycolysis operates as long as it is supplied with NAD that can pick up hydrogen atoms
D. glycolysis occurs in cytosol
![]() Q. 84 Select the incorrect match:
Q. 84 Select the incorrect match:
A. lampbrush chromosome – diplotene bivalents
B. polytene chromosome – oocytes of amphibians
C. submetacentric chromosome – L-shaped chromosomes
D. allosomes – sex chromosomes
![]() Q. 85 Which of the following terms describe human dentition?
Q. 85 Which of the following terms describe human dentition?
A. thecodont, diphyodont, homodont
B. pleurodont, diphyodont, heterodont
C. pleurodont, monophyodont, homodont
D. thecodont, diphyodont, heterodont
![]() Q. 86 Nissil bodies are mainly composed of
Q. 86 Nissil bodies are mainly composed of
A. proteins and lipids
B. free ribosomes and RER
C. nucleic acids and SER
D. DNA and RNA
![]() Q. 87 Many ribosomes may associate with a single mRNA to form multiple copies of a polypeptide simultaneously. Such strings of ribosomes are termed as:
Q. 87 Many ribosomes may associate with a single mRNA to form multiple copies of a polypeptide simultaneously. Such strings of ribosomes are termed as:
A. Polysome
B. Nucleosome
C. Plastidome
D. Polyhedral bodies
![]() Q. 88 Match the items given in the column I with those in column II (Given in figure) and select the correct option given below:
Q. 88 Match the items given in the column I with those in column II (Given in figure) and select the correct option given below:
A. a(iii), b(i), c(ii)
B. a(ii), b(i), c(iii)
C. a(i), b(ii), c(iii)
D. a(i), b(iii), c(ii)
![]() Q. 89 Match the item in the column I with the column II (Given in the figure) and select the correct option given below:
Q. 89 Match the item in the column I with the column II (Given in the figure) and select the correct option given below:
A. a(iii), b(ii), c(i), d(iv)
B. a(iv), b(iii), c(ii), d(i)
C. a(i), b(iv), c(ii), d(iii)
D. a(iii), b(i), c(iv0, d(ii)
![]() Q. 90 Which of the following options correctly represents the lung conditions in asthma and emphysema , respectively.
Q. 90 Which of the following options correctly represents the lung conditions in asthma and emphysema , respectively.
A. inflammation of bronchioles, decreased respiratory surface
B. decreased respiratory surface, inflammation of bronchioles
C. increased respiratory surface, inflammation of bronchioles
D. increased number of bronchioles, increased respiratory surface
![]() Q. 91 The stage during which separation of the paired homologous chromosomes begins is
Q. 91 The stage during which separation of the paired homologous chromosomes begins is
A. Pachytene
B. Zygotene
C. Diakinesis
D. Diplotene
![]() Q. 92 Which of the following is true for nucleolus?
Q. 92 Which of the following is true for nucleolus?
A. Larger nucleoli are present in dividing cells.
B. It is a site for active ribosomal RNA synthesis.
C. It takes part in spindle formation.
D. It is a membrane-bound structure.
![]() Q. 93 Stomatal movement is not affected by
Q. 93 Stomatal movement is not affected by
A. Temperature
B. CO₂ concentration
C. O₂ concentration
D. Light
![]() Q. 94 Which among the following is not a prokaryote ?
Q. 94 Which among the following is not a prokaryote ?
A. Saccharomyces
B. Oscillatoria
C. Nostoc
D. Mycobacterium
![]() Q. 95 Which of the following is not a product of light reaction of photosynthesis ?
Q. 95 Which of the following is not a product of light reaction of photosynthesis ?
A. ATP
B. Oxygen
C. NAPDH
D. NADH
![]() Q. 96 Stomata in grass leaf are
Q. 96 Stomata in grass leaf are
A. Dumb-bell shaped
B. Barrel shaped
C. Rectangular
D. Kidney shaped
![]() Q. 97 The Golgi complex participates in
Q. 97 The Golgi complex participates in
A. Fatty acid breakdown
B. Activation of amino acid
C. Respiration in bacteria
D. Formation of secretory vesicles
![]() Q. 98 The two functional groups characteristic of sugars are
Q. 98 The two functional groups characteristic of sugars are
A. hydroxyl and methyl
B. carbonyl and hydroxyl
C. carbonyl and phosphate
D. carbonyl and methyl
![]() Q. 99 A ‘new’ variety of rice was patented by a foreign company, though such varieties have been present in India for a long time. This is related to
Q. 99 A ‘new’ variety of rice was patented by a foreign company, though such varieties have been present in India for a long time. This is related to
A. Co-667
B. Basmati
C. Lerma Rojo
D. Sharbati Sonora
![]() Q. 100 Select the correct match:
Q. 100 Select the correct match:
A. Ribozyme – Nucleic acid
B. G. Mendel – Transformation
C. T.H. Morgan – Transduction
D. F₂ x Recessive parent – Dihybrid cross
![]() Q. 101 Which of the following is commonly used as a vector for introducing a DNA fragment in human lymphocytes ?
Q. 101 Which of the following is commonly used as a vector for introducing a DNA fragment in human lymphocytes ?
A. Retrovirus
B. pBR 322
C. λ phage
D. Ti plasmid
![]() Q. 102 In India, the organisation responsible for assessing the safety of introducing genetically modified organisms for public use is
Q. 102 In India, the organisation responsible for assessing the safety of introducing genetically modified organisms for public use is
A. Indian Council of Medical Research (ICMR)
B. Genetic Engineering Appraisal Committee (GEAC)
C. Research Committee on Genetic Manipulation (RCGM)
D. Council for Scientific and Industrial Research (CSIR)
![]() Q. 103 The correct order of steps in Polymerase Chain Reaction (PCR) is
Q. 103 The correct order of steps in Polymerase Chain Reaction (PCR) is
A. Extension, Denaturation, Annealing
B. Denaturation, Annealing, Extension
C. Denaturation, Extension, Annealing
D. Annealing, Extension, Denaturation
![]() Q. 104 Use of bioresources by multinational companies and organisations without authorisation from the concerned country and its people is called
Q. 104 Use of bioresources by multinational companies and organisations without authorisation from the concerned country and its people is called
A. Bio-infringement
B. Bioexploitation
C. Biodegradation
D. Biopiracy
![]() Q. 105 Winged pollen grains are present in
Q. 105 Winged pollen grains are present in
A. Mustard
B. Pinus
C. Mango
D. Cycas
![]() Q. 106 After karyogamy followed by meiosis, spores are produced exogenously in
Q. 106 After karyogamy followed by meiosis, spores are produced exogenously in
A. Neurospora
B. Saccharomyces
C. Agaricus
D. Alternaria
![]() Q. 107 Which one is wrongly matched ?
Q. 107 Which one is wrongly matched ?
A. Uniflagellate gametes – Polysiphonia
B. Unicellular organism – Chlorella
C. Gemma cups – Marchantia
D. Biflagellate zoospores – Brown algae
![]() Q. 108 Match the items given in Column I with those in Column II (Given in figure) and select the correct option given below:
Q. 108 Match the items given in Column I with those in Column II (Given in figure) and select the correct option given below:
A. a → i, b → iv, c → iii, d → ii
B. a → iii, b → iv, c → i, d → ii
C. a → ii, b → iv, c → iii, d → i
D. a → iii, b → ii, c → i, d → iv
![]() Q. 109 What is the role of NAD⁺ in cellular respiration ?
Q. 109 What is the role of NAD⁺ in cellular respiration ?
A. It functions as an enzyme.
B. It is the final electron acceptor for anaerobic respiration.
C. It is a nucleotide source for ATP synthesis.
D. It functions as an electron carrier.
![]() Q. 110 Oxygen is not produced during photosynthesis by
Q. 110 Oxygen is not produced during photosynthesis by
A. Green sulphur bacteria
B. Chara
C. Cycas
D. Nostoc
![]() Q. 111 Double fertilization is
Q. 111 Double fertilization is
A. Fusion of two male gametes of a pollen tube with two different eggs
B. Syngamy and triple fusion
C. Fusion of two male gametes with one egg
D. Fusion of one male gamete with two polar nuclei
![]() Q. 112 In which of the following forms is iron absorbed by plants ?
Q. 112 In which of the following forms is iron absorbed by plants ?
A. Ferric
B. Both ferric and ferrous
C. Free element
D. Ferrous
![]() Q. 113 Which of the following elements is responsible for maintaining turgor in cells?
Q. 113 Which of the following elements is responsible for maintaining turgor in cells?
A. Magnesium
B. Calcium
C. Potassium
D. Sodium
![]() Q. 114 Which one of the following plants shows a very close relationship with a species of moth, where none of the two can complete its life cycle without the other ?
Q. 114 Which one of the following plants shows a very close relationship with a species of moth, where none of the two can complete its life cycle without the other ?
A. Hydrilla
B. Voila
C. Banana
D. Yucca
![]() Q. 115 Pollen grains can be stored for several years in liquid nitrogen having a temperature of
Q. 115 Pollen grains can be stored for several years in liquid nitrogen having a temperature of
A. -120°C
B. -160°C
C. -196°C
D. -80°C
![]() Q. 116 Niche can be defined as:
Q. 116 Niche can be defined as:
A. All the biological factors in the organism’s environment
B. The functional role played by the organism where it lives
C. The range of temperature that the organism needs to live
D. The physical space where an organism lives
![]() Q. 117 Which of the following is a secondary pollutant ?
Q. 117 Which of the following is a secondary pollutant ?
A. CO
B. O₃
C. SO₂
D. CO₂
![]() Q. 118 World Ozone Day is celebrated on
Q. 118 World Ozone Day is celebrated on
A. 5th June
B. 22nd April
C. 16th September
D. 21st April
![]() Q. 119 In stratosphere, which of the following elements acts as a catalyst in degradation of ozone and release of molecular oxygen ?
Q. 119 In stratosphere, which of the following elements acts as a catalyst in degradation of ozone and release of molecular oxygen ?
A. Carbon
B. Oxygen
C. Fe
D. Cl
![]() Q. 120 What type of ecological pyramid would be obtained with the following data ?
Q. 120 What type of ecological pyramid would be obtained with the following data ?
Secondary consumer : 120 g
Primary consumer: 60 g
Primary producer: 10 g
A. Inverted pyramid of biomass
B. Upright pyramid of biomass
C. Upright pyramid of numbers
D. Pyramid of energy
![]() Q. 121 Natality refers to
Q. 121 Natality refers to
A. Death rate
B. Number of individuals entering a habitat
C. Number of individuals leaving a habitat
D. Birth rate
![]() Q. 122 Which of the following has proved helpful in preserving pollen as fossils ?
Q. 122 Which of the following has proved helpful in preserving pollen as fossils ?
A. Pollenkitt
B. Sporopollenin
C. Oil Content
D. Cellulosic intine
![]() Q. 123 Which of the following pairs is wrongly matched ?
Q. 123 Which of the following pairs is wrongly matched ?
A. Starch synthesis in pea : Multiple alleles
B. T.H. Morgan : Linkage
C. XO type sex determination : Grasshopper
D. ABO blood grouping : Co-dominance
![]() Q. 124 Select the correct match:
Q. 124 Select the correct match:
A. Alec Jeffreys – Streptococcus pneumoniae
B. Francois Jacob and Jacques Monod – Lac operon
C. Matthew Meselson and F. Stahl – Pisum sativum
D. Alfred Hershey and Martha Chase – TMV
![]() Q. 125 Which of the following flowers only once in its lifetime ?
Q. 125 Which of the following flowers only once in its lifetime ?
A. Bamboo species
B. Papaya
C. Mango
D. Jackfruit
![]() Q. 126 Select the correct statement:
Q. 126 Select the correct statement:
A. Franklin Stahl coined the term “linkage”.
B. Transduction was discovered by S. Altman.
C. Spliceosomes take part in translation.
D. Punnett square was developed by a British scientist.
![]() Q. 127 Offsets are produced by
Q. 127 Offsets are produced by
A. Meiotic divisions
B. Parthenogenesis
C. Parthenocarpy
D. Mitotic divisions
![]() Q. 128 The experimental proof for semiconservative replication of DNA was first shown in a
Q. 128 The experimental proof for semiconservative replication of DNA was first shown in a
A. Fungus
B. Virus
C. Plant
D. Bacterium
![]() Q. 129 Select the wrong statement:
Q. 129 Select the wrong statement:
A. Cell wall is present in members of Fungi and Plantae.
B. Mitochondria are the powerhouse of the cell in all kingdoms except Monera.
C. Pseudopodia are locomotory and feeding structures in Sporozoans.
D. Mushrooms belong to Basidiomycetes.
![]() Q. 130 Casparian strips occur in
Q. 130 Casparian strips occur in
A. Epidermis
B. Endodermis
C. Cortex
D. Pericycle
![]() Q. 131 Which of the following statements is correct ?
Q. 131 Which of the following statements is correct ?
A. Ovules are not enclosed by ovary wall in gymnosperms.
B. Stems are usually unbranched in both Cycas and Cedrus.
C. Horsetails are gymnosperms.
D. Selaginella is heterosporous, while Salvinia is homosporous
![]() Q. 132 Pneumatophores occur in
Q. 132 Pneumatophores occur in
A. Halophytes
B. Submerged hydrophytes
C. Carnivorous plants
D. Free-floating hydrophytes
![]() Q. 133 Sweet potato is a modified
Q. 133 Sweet potato is a modified
A. Stem
B. Rhizome
C. Tap root
D. Adventitious root
![]() Q. 134 Secondary xylem and phloem in dicot stem are produced by
Q. 134 Secondary xylem and phloem in dicot stem are produced by
A. Apical meristems
B. Axillary meristems
C. Phellogen
D. Vascular cambium
![]() Q. 135 Plants having little or no secondary growth are
Q. 135 Plants having little or no secondary growth are
A. Grasses
B. Cycads
C. Conifers
D. Deciduous angiosperms
![]() Q. 136 The power radiated by a black body is P and it radiates maximum energy at wavelength, λ0. If the temperature of the black body is now changed so that it radiates maximum energy at wavelength 3/4λ0 , the power radiated by it becomes nP.The value of n is
Q. 136 The power radiated by a black body is P and it radiates maximum energy at wavelength, λ0. If the temperature of the black body is now changed so that it radiates maximum energy at wavelength 3/4λ0 , the power radiated by it becomes nP.The value of n is
A. 3/4
B. 81/256
C. 256/81
D. 4/3
![]() Q. 137 Two wires are made of the same material and have the same volume. The first wire has cross-sectional area A and the second wire has cross-sectional area 3A. If the length of the first wire is increased by ΔI on applying a force F, how much force is needed to stretch the second wire by the same amount ?
Q. 137 Two wires are made of the same material and have the same volume. The first wire has cross-sectional area A and the second wire has cross-sectional area 3A. If the length of the first wire is increased by ΔI on applying a force F, how much force is needed to stretch the second wire by the same amount ?
A. 9 F
B. F
C. 4 F
D. 6 F
![]() Q. 138 A sample of 0.1 g of water at 100°C and normal pressure (1.013×10⁵ Nm⁻²) requires 54 cal of heat energy to convert to steam at 100°C. If the volume of the steam produced is 167.1 cc, the change in internal energy of the sample, is
Q. 138 A sample of 0.1 g of water at 100°C and normal pressure (1.013×10⁵ Nm⁻²) requires 54 cal of heat energy to convert to steam at 100°C. If the volume of the steam produced is 167.1 cc, the change in internal energy of the sample, is
A. 104.3 J
B. 84.5 J
C. 42.2 J
D. 208.7 J
![]() Q. 139 A small sphere of radius ‘r’ falls from rest in a viscous liquid. As a result, heat is produced due to viscous force. The rate of production of heat when the sphere attains its terminal velocity, is proportional to
Q. 139 A small sphere of radius ‘r’ falls from rest in a viscous liquid. As a result, heat is produced due to viscous force. The rate of production of heat when the sphere attains its terminal velocity, is proportional to
A. r³
B. r⁴
C. r⁵
D. r²
![]() Q. 140 An electron falls from rest through a vertical distance h in a uniform and vertically upward directed electric field E. The direction of electric field is now reversed, keeping its magnitude the same. A proton is allowed to fall from rest in it through the same vertical distance h. The time of fall of the electron, in comparison to the time of fall of the proton is
Q. 140 An electron falls from rest through a vertical distance h in a uniform and vertically upward directed electric field E. The direction of electric field is now reversed, keeping its magnitude the same. A proton is allowed to fall from rest in it through the same vertical distance h. The time of fall of the electron, in comparison to the time of fall of the proton is
A. smaller
B. equal
C. 10 times greater
D. 5 times greater
![]() Q. 141 A pendulum is hung from the roof of a sufficiently high building and is moving freely to and fro like a simple harmonic oscillator. The acceleration of the bob of the pendulum is 20 m/s² at a distance of 5 m from the mean position. The time period of oscillation is
Q. 141 A pendulum is hung from the roof of a sufficiently high building and is moving freely to and fro like a simple harmonic oscillator. The acceleration of the bob of the pendulum is 20 m/s² at a distance of 5 m from the mean position. The time period of oscillation is
A. 2 π s
B. 1 s
C. 2 s
D. π s
![]() Q. 142 The electrostatic force between the metal plate of an isolated parallel plate capacitor C having charge Q and area A, is
Q. 142 The electrostatic force between the metal plate of an isolated parallel plate capacitor C having charge Q and area A, is
A. independent of the distance between the plates.
B. inversely proportional to the distance between the plates.
C. proportional to the square root of the distance between the plates.
D. linearly proportional to the distance between the plates.
![]() Q. 143 A tuning fork is used to produce resonance in glass tube. The length of the air column in the tube can be adjusted by a variable piston. At room temperature of 27°C two successive resonances are produced at 20 cm and 73 cm of column length. If the frequency of the tuning fork is 320 Hz, the velocity of sound in air at 27°C
Q. 143 A tuning fork is used to produce resonance in glass tube. The length of the air column in the tube can be adjusted by a variable piston. At room temperature of 27°C two successive resonances are produced at 20 cm and 73 cm of column length. If the frequency of the tuning fork is 320 Hz, the velocity of sound in air at 27°C
A. 330 m/s
B. 300 m/s
C. 350 m/s
D. 339 m/s
![]() Q. 144 The ratio of kinetic energy to the total energy of an electron in a Bohr orbit of the hydrogen atom. is
Q. 144 The ratio of kinetic energy to the total energy of an electron in a Bohr orbit of the hydrogen atom. is
A. 1 : 1
B. 1 : -2
C. 2 : -1
D. 1 : -1
![]() Q. 145 When the light of frequency 2v0 (where v0 is threshold frequency), is incident on a metal plate, the maximum velocity of electrons emitted is v1. When the frequency of the incident radiation is increased to 5v0, the maximum velocity of electrons emitted from the same plate is v2. The ratio of v1 to v2 is
Q. 145 When the light of frequency 2v0 (where v0 is threshold frequency), is incident on a metal plate, the maximum velocity of electrons emitted is v1. When the frequency of the incident radiation is increased to 5v0, the maximum velocity of electrons emitted from the same plate is v2. The ratio of v1 to v2 is
A. 1 : 2
B. 2 : 1
C. 4 : 1
D. 1 : 4
![]() Q. 146 For a radioactive material, half-life is 10 minutes. If initially there are 600 number of nuclei, the time taken (in minutes) for the disintegration of 450 nuclei is
Q. 146 For a radioactive material, half-life is 10 minutes. If initially there are 600 number of nuclei, the time taken (in minutes) for the disintegration of 450 nuclei is
A. 20
B. 15
C. 30
D. 10
![]() Q. 147 An electron of mass m with an initial velocity V⃗ =V₀î(V₀ > 0) enters an electric field E⃗ = −E₀î (E₀ = constant > 0) at t=0. If λ₀ is its de-Broglie wavelength initially, then its de-Broglie wavelength at time t is
Q. 147 An electron of mass m with an initial velocity V⃗ =V₀î(V₀ > 0) enters an electric field E⃗ = −E₀î (E₀ = constant > 0) at t=0. If λ₀ is its de-Broglie wavelength initially, then its de-Broglie wavelength at time t is
A. λ₀/(1 + (eE₀/mV₀)t)
B. λ₀
C. λ₀t
D. λ₀(1 + (eE₀/mV₀)t)
![]() Q. 148 An inductor 20 mH, a capacitor 100 μF and a resistor 50 Ω are connected in series across a source of emf, V = 10 sin 314 t. The power loss in the circuit is
Q. 148 An inductor 20 mH, a capacitor 100 μF and a resistor 50 Ω are connected in series across a source of emf, V = 10 sin 314 t. The power loss in the circuit is
A. 0.79 W
B. 1.13 W
C. 2.74 W
D. 0.43 W
![]() Q. 149 A metallic rod of mass per unit length 0.5 kg m⁻¹ is lying horizontally on a smooth inclined plane which makes an angle of 30° with the horizontal. The rod is not allowed to slide down by flowing a current through it when a magnetic field of induction 0.25 T is acting on it in the vertical direction. The current flowing in the rod to keep it stationary is
Q. 149 A metallic rod of mass per unit length 0.5 kg m⁻¹ is lying horizontally on a smooth inclined plane which makes an angle of 30° with the horizontal. The rod is not allowed to slide down by flowing a current through it when a magnetic field of induction 0.25 T is acting on it in the vertical direction. The current flowing in the rod to keep it stationary is
A. 7.14 A
B. 11.32 A
C. 14.76 A
D. 5.98 A
![]() Q. 150 A thin diamagnetic rod is placed vertically between the poles of an electromagnet. When the current in the electromagnet is switched on, then the diamagnetic rod is pushed up, out of the horizontal magnetic field. Hence the rod gains gravitational potential energy. The work required to do this comes from
Q. 150 A thin diamagnetic rod is placed vertically between the poles of an electromagnet. When the current in the electromagnet is switched on, then the diamagnetic rod is pushed up, out of the horizontal magnetic field. Hence the rod gains gravitational potential energy. The work required to do this comes from
A. the current source
B. the induced electric field due to the changing magnetic field
C. the lattice structure of the material of the rod
D. the magnetic field
![]() Q. 151 Current sensitivity of a moving coil galvanometer is 5 div/mA and its voltage sensitivity (angular deflection per unit voltage applied) is 20 div/V. The resistance of the galvanometer is
Q. 151 Current sensitivity of a moving coil galvanometer is 5 div/mA and its voltage sensitivity (angular deflection per unit voltage applied) is 20 div/V. The resistance of the galvanometer is
A. 40 Ω
B. 500 Ω
C. 250 Ω
D. 25 Ω
![]() Q. 152 A solid sphere is in rolling motion. In rolling motion a body possesses translational kinetic energy (Kt) as well as rotational kinetic energy (Kr) simultaneously. The ratio Kt : (Kt + Kr) for the sphere is
Q. 152 A solid sphere is in rolling motion. In rolling motion a body possesses translational kinetic energy (Kt) as well as rotational kinetic energy (Kr) simultaneously. The ratio Kt : (Kt + Kr) for the sphere is
A. 7 : 10
B. 2 : 5
C. 10 : 7
D. 5 : 7
![]() Q. 153 The kinetic energies of a planet in an elliptical orbit about the Sun, at positions A, B and C are KA, KB and KC respectively. AC is the major axis and SB is perpendicular to AC at the position of the Sun S as shown in the figure. Then
Q. 153 The kinetic energies of a planet in an elliptical orbit about the Sun, at positions A, B and C are KA, KB and KC respectively. AC is the major axis and SB is perpendicular to AC at the position of the Sun S as shown in the figure. Then
A. KA < KB < KC
B. KB > KA > KC
C. KB < KA < KC
D. KA > KB > KC
![]() Q. 154 If the mass of the Sun were ten times smaller and the universal gravitational constant were ten times larger in magnitude, which of the following is not correct ?
Q. 154 If the mass of the Sun were ten times smaller and the universal gravitational constant were ten times larger in magnitude, which of the following is not correct ?
A. Raindrops will fall faster
B. g’ on the Earth will not change
C. Time period of a simple pendulum on the Earth would decrease.
D. Walking on the ground would become more difficult.
![]() Q. 155 A solid sphere is rotating freely about its symmetry axis in free space. The radius of the sphere is increased keeping its mass same. Which of the following physical quantities would remain constant for the sphere ?
Q. 155 A solid sphere is rotating freely about its symmetry axis in free space. The radius of the sphere is increased keeping its mass same. Which of the following physical quantities would remain constant for the sphere ?
A. Angular velocity
B. Angular momentum
C. Rotational kinetic energy
D. Moment of inertia
![]() Q. 156 Unpolarised light is incident from air on a plane surface of a material of refractive index ′μ′. At a particular angle of incidence ′i′, it is found that the reflected and refracted rays are perpendicular to each other. Which of the following options is correct for this situation ?
Q. 156 Unpolarised light is incident from air on a plane surface of a material of refractive index ′μ′. At a particular angle of incidence ′i′, it is found that the reflected and refracted rays are perpendicular to each other. Which of the following options is correct for this situation ?
A. Reflected light is polarised with its electric vector parallel to the plane of incidence
B. i = tan⁻¹(1/μ)
C. i = sin⁻¹(1/μ)
D. Reflected light is polarised with its electric vector perpendicular to the plane of incidence
![]() Q. 157 In Young’s double slit experiment the separation d between the slits is 2 mm, the wavelength λ of the light used is 5896 Å and distance D between the screen and slits is 100 cm. It is found that the angular width of the fringes is 0.20°. To increase the fringe angular width to 0.21° (with same λ and D) the separation between the slits needs to be changed to
Q. 157 In Young’s double slit experiment the separation d between the slits is 2 mm, the wavelength λ of the light used is 5896 Å and distance D between the screen and slits is 100 cm. It is found that the angular width of the fringes is 0.20°. To increase the fringe angular width to 0.21° (with same λ and D) the separation between the slits needs to be changed to
A. 1.8 mm
B. 1.7 mm
C. 2.1mm
D. 1.9 mm
![]() Q. 158 An astronomical refracting telescope will have large angular magnification and high angular resolution, when it has an objective lens of
Q. 158 An astronomical refracting telescope will have large angular magnification and high angular resolution, when it has an objective lens of
A. small focal length and large diameter
B. small focal length and small diameter
C. large focal length and large diameter
D. large focal length and small diameter
![]() Q. 159 A carbon resistor of (47±4.7) kΩ is to be marked with rings of different colours for its identification. The colour code sequence will be
Q. 159 A carbon resistor of (47±4.7) kΩ is to be marked with rings of different colours for its identification. The colour code sequence will be
A. Violet – Yellow – Orange – Silver
B. Green – Orange- Violet- Gold
C. Yellow – Green – Violet – Gold
D. Yellow – Violet – Orange – Silver
![]() Q. 160 A set of ‘n’ equal resistors, of value R each, are connected in series to a battery of emf E and internal resistance ‘R’ The current drawn is I. Now, the ‘n’ resistors are connected in parallel to the same battery. Then the current drawn from battery becomes 10 I. The value of ‘n’ is
Q. 160 A set of ‘n’ equal resistors, of value R each, are connected in series to a battery of emf E and internal resistance ‘R’ The current drawn is I. Now, the ‘n’ resistors are connected in parallel to the same battery. Then the current drawn from battery becomes 10 I. The value of ‘n’ is
A. 10
B. 9
C. 20
D. 11
![]() Q. 161 A battery consists of a variable number ‘n’ of identical cells (having internal resistance ‘r’ each) which are connected in series. The terminals of the battery are short-circuited and the current I is measured. Which of the graphs shows (among (1), (2), (3), (4)) the correct relationship between I and n ?
Q. 161 A battery consists of a variable number ‘n’ of identical cells (having internal resistance ‘r’ each) which are connected in series. The terminals of the battery are short-circuited and the current I is measured. Which of the graphs shows (among (1), (2), (3), (4)) the correct relationship between I and n ?
A. 1
B. 2
C. 3
D. 4
![]() Q. 162 A body initially at rest and sliding along a frictionless track from a height h (as shown in the figure) just completes a vertical circle of diameter AB = D. The height h is equal to
Q. 162 A body initially at rest and sliding along a frictionless track from a height h (as shown in the figure) just completes a vertical circle of diameter AB = D. The height h is equal to
A. 3/2 D
B. 5/4 D
C. 7/5 D
D. D
![]() Q. 163 Three objects, A : (a solid sphere), B : (a thin circular disk) and C : (a circular ring), each have the same mass M and radius R. They all spin with the same angular speed ω to about their own symmetry axes. The amounts of work (W) required to bring them to rest, would satisfy the relation
Q. 163 Three objects, A : (a solid sphere), B : (a thin circular disk) and C : (a circular ring), each have the same mass M and radius R. They all spin with the same angular speed ω to about their own symmetry axes. The amounts of work (W) required to bring them to rest, would satisfy the relation
A. WC > WB > WA
B. WA > WC > WB
C. WB > WA > WC
D. WA > WB > WC
![]() Q. 164 Which one of the following statements is incorrect ?
Q. 164 Which one of the following statements is incorrect ?
A. Rolling friction is smaller than sliding friction
B. Coefficient of sliding friction has dimensions of length
C. Frictional force opposes the relative motion
D. Limiting value of static friction is directly proportional to normal reactions
![]() Q. 165 A moving block having mass m, collides with another stationary block having mass 4m. The lighter block comes to rest after collision. When the initial velocity of the lighter block is v, then the value of coefficient of restitution (e) will be
Q. 165 A moving block having mass m, collides with another stationary block having mass 4m. The lighter block comes to rest after collision. When the initial velocity of the lighter block is v, then the value of coefficient of restitution (e) will be
A. 0.5
B. 0.4
C. 0.8
D. 0.25
![]() Q. 166 An em wave is propagating in a medium with a velocity V⃗ = Vî. The instantaneous oscillating electric field of this em wave is along +y axis. Then the direction of oscillating magnetic field of the em wave will be along :-
Q. 166 An em wave is propagating in a medium with a velocity V⃗ = Vî. The instantaneous oscillating electric field of this em wave is along +y axis. Then the direction of oscillating magnetic field of the em wave will be along :-
A. –z direction
B. –x direction
C. –y direction
D. +z direction
![]() Q. 167 The refractive index of the material of a prism is √2 and the angle of the prism is 30°. One of the two refracting surfaces of the prism is made a mirror inwards, by silver coating. A beam of monochromatic light entering the prism from the other face will retrace its path (after reflection from the silvered surface) if its angle of incidence on the prism is :-
Q. 167 The refractive index of the material of a prism is √2 and the angle of the prism is 30°. One of the two refracting surfaces of the prism is made a mirror inwards, by silver coating. A beam of monochromatic light entering the prism from the other face will retrace its path (after reflection from the silvered surface) if its angle of incidence on the prism is :-
A. 60°
B. zero
C. 30°
D. 45°
![]() Q. 168 The magnetic potential energy stored in a certain inductor is 25 mJ, when the current in the inductor is 60 mA. This inductor is of inductance
Q. 168 The magnetic potential energy stored in a certain inductor is 25 mJ, when the current in the inductor is 60 mA. This inductor is of inductance
A. 0.138 H
B. 13.89 H
C. 1.389 H
D. 138.88 H
![]() Q. 169 An object is placed at a distance of 40 cm from a concave mirror of focal length 15 cm. If the object is displaced through a distance of 20 cm towards the mirror, the displacement of the image will be:-
Q. 169 An object is placed at a distance of 40 cm from a concave mirror of focal length 15 cm. If the object is displaced through a distance of 20 cm towards the mirror, the displacement of the image will be:-
A. 30 cm away the mirror
B. 36 cm towards the mirror
C. 30 cm towards the mirror
D. 36 cm away the mirror
![]() Q. 170 In the circuit shown in the figure, the input voltage Vi is 20 V, VBE = 0 and VCE = 0. The values of IB, IC and β are given by :-
Q. 170 In the circuit shown in the figure, the input voltage Vi is 20 V, VBE = 0 and VCE = 0. The values of IB, IC and β are given by :-
A. IB = 40 μA, IC = 10 mA, β = 250
B. IB = 40 μA, IC = 5 mA, β = 125
C. IB = 20 μA, IC = 5 mA, β = 250
D. IB = 25 μA, IC = 5 mA, β = 200
![]() Q. 171 In a p-n junction diode , change in temperature due to heating
Q. 171 In a p-n junction diode , change in temperature due to heating
A. affects only reverse resistance
B. affects the overall V-I characteristics of p-n junction
C. does not affect resistance of p-n junction
D. affects only forward resistance
![]() Q. 172 In the combination of the following gates output Y can be written in terms of input A and B as :
Q. 172 In the combination of the following gates output Y can be written in terms of input A and B as :
A. A
B. B
C. C
D. D
![]() Q. 173 A toy car with charge q moves on a frictionless horizontal plane surface under the influence of a uniform electric field E⃗ . Due to the force q E⃗ , its velocity increases from 0 to 6 m/s in one second duration. At that instant the direction of the field is reversed. The car continues to move for two more seconds under the influence of this field. The average velocity and the average speed of the toy car between 0 to 3 seconds are respectively
Q. 173 A toy car with charge q moves on a frictionless horizontal plane surface under the influence of a uniform electric field E⃗ . Due to the force q E⃗ , its velocity increases from 0 to 6 m/s in one second duration. At that instant the direction of the field is reversed. The car continues to move for two more seconds under the influence of this field. The average velocity and the average speed of the toy car between 0 to 3 seconds are respectively
A. 2 m/s , 4 m/s
B. 1.5 m/s , 3 m/s
C. 1 m/s , 3.5 m/s
D. 1 m/s , 3 m/s
![]() Q. 174 A block of mass m is placed on a smooth inclined wedge ABC of inclination θ as shown in the figure. The wedge is given an acceleration ‘a’ towards right .The relation between a and θ for the block to remain stationary on the wedge is
Q. 174 A block of mass m is placed on a smooth inclined wedge ABC of inclination θ as shown in the figure. The wedge is given an acceleration ‘a’ towards right .The relation between a and θ for the block to remain stationary on the wedge is
A. a= g/cosec θ
B. a= g tan θ
C. a= g cos θ
D. a= g/sin θ
![]() Q. 175 A student measured the diameter of the small steel ball using the screw gauge of least count 0.001 cm .The main scale reading is 5 mm and zero of circular scale division coincides with 25 divisions above the reference level. If screw gauge has a zero error of -0.004 cm , the correct diameter of the ball is :
Q. 175 A student measured the diameter of the small steel ball using the screw gauge of least count 0.001 cm .The main scale reading is 5 mm and zero of circular scale division coincides with 25 divisions above the reference level. If screw gauge has a zero error of -0.004 cm , the correct diameter of the ball is :
A. 0.521 cm
B. 0.529 cm
C. 0.053 cm
D. 0.525 cm
![]() Q. 176 The moment of the force F = 4î + 5ĵ – 6k̂ at (2,0,-3) about the point (2, -2,-2) is given by
Q. 176 The moment of the force F = 4î + 5ĵ – 6k̂ at (2,0,-3) about the point (2, -2,-2) is given by
A. -8î – 4ĵ – 7k̂
B. -7î – 4ĵ – 8k̂
C. -7î – 8ĵ – 4k̂
D. -4î – ĵ – 8k̂
![]() Q. 177 The volume (V) of a monatomic gas varies with its temperature (T), as shown in the graph. The ratio of work done by the gas, to the heat absorbed by it, when it undergoes a change from state A to state B, is
Q. 177 The volume (V) of a monatomic gas varies with its temperature (T), as shown in the graph. The ratio of work done by the gas, to the heat absorbed by it, when it undergoes a change from state A to state B, is
A. 2/5
B. 2/7
C. 1/3
D. 2/3
![]() Q. 178 The fundamental frequency in an open organ pipe is equal to the third harmonic of a closed organ pipe. If the length of the closed organ pipe is 20 cm , the length of the open organ pipe is :
Q. 178 The fundamental frequency in an open organ pipe is equal to the third harmonic of a closed organ pipe. If the length of the closed organ pipe is 20 cm , the length of the open organ pipe is :
A. 13.2 cm
B. 16 cm
C. 12.5 cm
D. 8 cm
![]() Q. 179 The efficiency of an ideal heat engine working between the freezing point and boiling point of water, is:
Q. 179 The efficiency of an ideal heat engine working between the freezing point and boiling point of water, is:
A. 26.8 %
B. 12.5%
C. 6.25%
D. 20%
![]() Q. 180 At what temperature will the rms speed of oxygen molecules become just sufficient from escaping from Earth’s atmosphere?
Q. 180 At what temperature will the rms speed of oxygen molecules become just sufficient from escaping from Earth’s atmosphere?
Given: Mass of oxygen molecule (m) = 2.76 x 10⁻²⁶ kg
Boltzmann’s constant kB = 1.38 x 10⁻²³ J K⁻¹)
A. 2.508 x 10⁴ K
B. 1.254 x 10⁴ K
C. 5.016 x 10⁴ K
D. 8.360 x 10⁴ K
| Question | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Answer | A | B | D | A | C | C | B | B | A | A |
| Question | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Answer | B | C | A | A | A | D | D | B | A | B |
| Question | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Answer | B | D | C | A | B | C | C | D | B | B |
| Question | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 |
| Answer | C | D | A | B | A | B | B | D | A | C |
| Question | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 |
| Answer | D | B | A | C | D | D | A | B | B | C |
| Question | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 |
| Answer | A | D | A | B | A | C | C | D | A | A |
| Question | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 |
| Answer | D | D | D | B | C | A | C | A | B | A |
| Question | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 |
| Answer | C | D | B | D | B | D | B | A | C | D |
| Question | 81 | 82 | 83 | 84 | 85 | 86 | 87 | 88 | 89 | 90 |
| Answer | C | B | B | B | D | B | A | A | D | A |
| Question | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 100 |
| Answer | D | B | C | A | D | A | D | B | B | A |
| Question | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | 109 | 110 |
| Answer | A | B | B | D | B | C | A | B | D | A |
| Question | 111 | 112 | 113 | 114 | 115 | 116 | 117 | 118 | 119 | 120 |
| Answer | B | A | C | D | C | B | B | C | D | A |
| Question | 121 | 122 | 123 | 124 | 125 | 126 | 127 | 128 | 129 | 130 |
| Answer | D | B | A | B | A | D | D | D | C | B |
| Question | 131 | 132 | 133 | 134 | 135 | 136 | 137 | 138 | 139 | 140 |
| Answer | A | A | D | D | A | C | A | D | C | A |
| Question | 141 | 142 | 143 | 144 | 145 | 146 | 147 | 148 | 149 | 150 |
| Answer | D | A | D | D | A | A | A | A | B | A |
| Question | 151 | 152 | 153 | 154 | 155 | 156 | 157 | 158 | 159 | 160 |
| Answer | C | D | D | B | B | D | D | C | D | A |
| Question | 161 | 162 | 163 | 164 | 165 | 166 | 167 | 168 | 169 | 170 |
| Answer | A | B | A | B | D | D | D | B | D | B |
| Question | 171 | 172 | 173 | 174 | 175 | 176 | 177 | 178 | 179 | 180 |
| Answer | B | D | D | B | B | B | A | A | A | D |