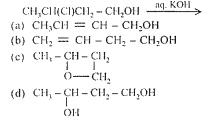![]() AIIMS UG 2010
AIIMS UG 2010
|
Section |
Questions |
Marks |
|
Physics |
60 Questions (1 – 60) |
60 |
|
Chemistry |
60 Questions (61 – 120) |
60 |
|
Biology |
60 Questions (121 – 180) |
60 |
|
General Knowledge |
20 Questions (181 – 200) |
20 |
![]() Q. 1 Transmission lines transmit a voltage of V volt to our houses from power stations, then the power P is supplied by them is proportional to :
Q. 1 Transmission lines transmit a voltage of V volt to our houses from power stations, then the power P is supplied by them is proportional to :
A. 1/V
B. V
C. V²
D. 1/V²
![]() Q. 2 Whenever a stream of electrons collides with a stream of photons, in the collision , which of the following is not conserved?
Q. 2 Whenever a stream of electrons collides with a stream of photons, in the collision , which of the following is not conserved?
A. linear momentum
B. total energy
C. no. of photons
D. no. of electrons
![]() Q. 3 The logic gate represented in following figure is:
Q. 3 The logic gate represented in following figure is:
A. OR gate
B. NOT gate
C. NAND gate
D. XOR gate
![]() Q. 4 for a person near point of vision is 10cm. Then the power of lens he must wear so as to have normal vision.should be
Q. 4 for a person near point of vision is 10cm. Then the power of lens he must wear so as to have normal vision.should be
A. +1 D
B. -1 D
C. +3 D
D. -3 D
![]() Q. 5 Two projectiles of same mass have their maximum kinetic energies in ratio 4:1 and ratio of their maximum heights is also 4: 1 then what is the ratio of their ranges?
Q. 5 Two projectiles of same mass have their maximum kinetic energies in ratio 4:1 and ratio of their maximum heights is also 4: 1 then what is the ratio of their ranges?
A. 2:1
B. 4:1
C. 8:1
D. 16:1
![]() Q. 6 An unchanged particle is moving with a velocity of v̅ is non uniform magnetic field as shown. velocity v̅ would be
Q. 6 An unchanged particle is moving with a velocity of v̅ is non uniform magnetic field as shown. velocity v̅ would be
A. maximum at A and B
B. minimum at A and B
C. maximum at M
D. same at all points
![]() Q. 7 which of the following is true regarding diamagnetic substances (symbols have their usual meaning)
Q. 7 which of the following is true regarding diamagnetic substances (symbols have their usual meaning)
A. χᵥ> 1, μᵣ > 1
B. χᵥ < 1, μᵣ > 1
C. χᵥ < 0, μᵣ < 1
D. χᵥ > 0, μᵣ < 1
![]() Q. 8 what is moment of inertia of a cylinder of radius r, along its height?
Q. 8 what is moment of inertia of a cylinder of radius r, along its height?
A. mr²
B. mr²/2
C. 2mr²/5
D. mr²/5
![]() Q. 9 A uniform string is vibrating with a fundamental frequency ‘f’. The new frequency, if its radius and length both are doubled would be:
Q. 9 A uniform string is vibrating with a fundamental frequency ‘f’. The new frequency, if its radius and length both are doubled would be:
A. 2f
B. 3f
C. f/4
D. f/3
![]() Q. 10 Two spherical soap bubbles of radii a and b vacuum coaleasce under isothermal
Q. 10 Two spherical soap bubbles of radii a and b vacuum coaleasce under isothermal
conditions. The resulting bubbles has a radius given by:
A. (a+b)/2
B. ab/(a+b)
C. √(a²+b²)
D. a + b
![]() Q. 11 What would be the voltage across C₃ ?
Q. 11 What would be the voltage across C₃ ?
A. (C₁ + C₂)V/C₁ + C₂ + C₃
B. C₁V/ C₁ + C₂ + C₃
C. C₂V/C₁ + C₂ + C₃
D. C₃V/C₁ + C₂ + C₃
![]() Q. 12 What would be maximum wavelength for Brackett series of hydrogen – spectrum?
Q. 12 What would be maximum wavelength for Brackett series of hydrogen – spectrum?
A. 74582 A⁰
B. 22790 A⁰
C. 40519 A⁰
D. 18753 A⁰
![]() Q. 13 what would be the radius of second orbit of He⁺ ion?
Q. 13 what would be the radius of second orbit of He⁺ ion?
A. 1.058 A⁰
B. 3.023 A⁰
C. 2.068 A⁰
D. 4.458 A⁰
![]() Q. 14 The position of a particle moving in the x-y plane at any time t is given by ; x = (3t² – 6t ) meters; y = (t²-2t) meters. select the correct statement.
Q. 14 The position of a particle moving in the x-y plane at any time t is given by ; x = (3t² – 6t ) meters; y = (t²-2t) meters. select the correct statement.
A. acceleration is zero at t = 0
B. velocity is zero t = 0
C. velocity is zero at t = 1s
D. velocity and acceleration of the particle are never zero
![]() Q. 15 Two masses M₁ = 5 kg and M₂ = 10kg are connected at the ends of an inextensible string passing over a firctionless pulley as shown. when the masses are released, then the acceleration of the masses will be:
Q. 15 Two masses M₁ = 5 kg and M₂ = 10kg are connected at the ends of an inextensible string passing over a firctionless pulley as shown. when the masses are released, then the acceleration of the masses will be:
A. g
B. g/2
C. g/3
D. g/4
![]() Q. 16 A block of mass m is pulled along a horizontal surface by applying a force at an angle θ with the horizontal. If the block travels with a uniform velocity has a displacement d and the coefficient of friction μ is , then the work done by the applied force is
Q. 16 A block of mass m is pulled along a horizontal surface by applying a force at an angle θ with the horizontal. If the block travels with a uniform velocity has a displacement d and the coefficient of friction μ is , then the work done by the applied force is
A. μmgd/cos θ + μ sin θ
B. μmgd cos θ/cos θ + μ sin θ
C. μmgd sin θ/ cos θ + μ sin θ
D. μmgd cos θ/ cos θ-μ sin θ
![]() Q. 17 pressure versus temperature graph of an ideal gas is as shown in figure. Density of the gas at point A is ρₒ . density at point B will be
Q. 17 pressure versus temperature graph of an ideal gas is as shown in figure. Density of the gas at point A is ρₒ . density at point B will be
A. 3ρₒ/4
B. 3ρₒ/2
C. 4ρₒ/3
D. 2ρₒ
![]() Q. 18 The latent heat of vaporisation of a substance is always:
Q. 18 The latent heat of vaporisation of a substance is always:
A. greater than its latent heat of fusion
B. greater than its latent heat of sublimation
C. equal to its latent heat of sublimation
D. less than its latent heat of fusion
![]() Q. 19 A reversible engine converts one -sixth of the heat input into work. when the temperature of the sink is reduced by 62⁰C, the efficiency of the engine is doubled. The temperature of the source and sink are:
Q. 19 A reversible engine converts one -sixth of the heat input into work. when the temperature of the sink is reduced by 62⁰C, the efficiency of the engine is doubled. The temperature of the source and sink are:
A. 99⁰ C,37⁰ C
B. 80⁰ C, 37⁰ C
C. 95⁰ C, 37⁰ C
D. 90⁰ C, 37⁰ C
![]() Q. 20 Graph of specific heat at constant volume for a mono atomic gas is:
Q. 20 Graph of specific heat at constant volume for a mono atomic gas is:
A. a
B. b
C. c
D. d
![]() Q. 21 In figure , a particle having mass m = 5 g and charge q’ = 2 x 10⁻⁹ C starts from rest at point a and moves in a straight line to point b. What is its speed v at point b?
Q. 21 In figure , a particle having mass m = 5 g and charge q’ = 2 x 10⁻⁹ C starts from rest at point a and moves in a straight line to point b. What is its speed v at point b?
A. 2.65 cm/s
B. 3.65 cm /s
C. 4.65 cm /s
D. 5.65 cm /s
![]() Q. 22 A galvanometer has a current sensitivity of 1mA per division . A variable shunt is connected across the galvanometer and the combination is put in series with a resistance of 500 ohms and cell of internal resistance 1 ohm. It gives a deflection of 5 division for shunt of 5 ohm and 20 division for shunt of 25 ohm. The emf of cell is :
Q. 22 A galvanometer has a current sensitivity of 1mA per division . A variable shunt is connected across the galvanometer and the combination is put in series with a resistance of 500 ohms and cell of internal resistance 1 ohm. It gives a deflection of 5 division for shunt of 5 ohm and 20 division for shunt of 25 ohm. The emf of cell is :
A. 47.1V
B. 57.1 V
C. 67.1 V
D. 77.1V
![]() Q. 23 A circular coil with a cross-sectional area of 4 cm² has 10 turns. It is placed at the centre of a long solenoid that has 15 turns/cm and a cross-sectional area of 10 cm², as shown in the figure. The axis of the coil coincides with the axis of the solenoid. what is their mutual inductance?
Q. 23 A circular coil with a cross-sectional area of 4 cm² has 10 turns. It is placed at the centre of a long solenoid that has 15 turns/cm and a cross-sectional area of 10 cm², as shown in the figure. The axis of the coil coincides with the axis of the solenoid. what is their mutual inductance?
A. 7.54 μH
B. 8.54 μH
C. 9.54 μH
D. 10.54 μH
![]() Q. 24 If K₁ and K₂ are maximum kinetic energies of photo electrons emitted when lights of wavelengths λ₁ and λ₂ respectively incident on a metallic surface. If λ₁ = 3λ₂,then
Q. 24 If K₁ and K₂ are maximum kinetic energies of photo electrons emitted when lights of wavelengths λ₁ and λ₂ respectively incident on a metallic surface. If λ₁ = 3λ₂,then
A. K₁ > K₂/3
B. K₁ < K₂/3
C. K₁ = 2K₂
D. K₂ = 2K₁
![]() Q. 25 Two radio active substances A and B have decay constants 5λ and λ respectively. At t = 0, they have the same number of nuclei of A to those of B will be (1/e²) after a time
Q. 25 Two radio active substances A and B have decay constants 5λ and λ respectively. At t = 0, they have the same number of nuclei of A to those of B will be (1/e²) after a time
A. 4λ
B. 2λ
C. 1/2λ
D. 1/4λ
![]() Q. 26 The intensity of gamma radiation from a given source is l. On passing through 36mm of lead, it is reduced to l/8. The thickness of lead which will reduce the intensity to l/2 will be:
Q. 26 The intensity of gamma radiation from a given source is l. On passing through 36mm of lead, it is reduced to l/8. The thickness of lead which will reduce the intensity to l/2 will be:
A. 18mm
B. 12mm
C. 6mm
D. 9mm
![]() Q. 27 An electric charge 10⁻¹ μC is placed at the origin (0,0) of (x-y) co-ordinate system. Two points A and B are situated at (√2,√2) and (2,0) respectively. The potential difference between the points A and B will be:
Q. 27 An electric charge 10⁻¹ μC is placed at the origin (0,0) of (x-y) co-ordinate system. Two points A and B are situated at (√2,√2) and (2,0) respectively. The potential difference between the points A and B will be:
A. 4.5 volt
B. 9 volt
C. zero
D. 2 volt
![]() Q. 28 If the energy, E = Gⁿ₁hⁿ₂cⁿ₃ , where G is the universal gravitational constant, h is the planks constant and c is the velocity of light, then the values of ⁿ₁, ⁿ₂, ⁿ₃ are respectively:
Q. 28 If the energy, E = Gⁿ₁hⁿ₂cⁿ₃ , where G is the universal gravitational constant, h is the planks constant and c is the velocity of light, then the values of ⁿ₁, ⁿ₂, ⁿ₃ are respectively:
A. -1/2,1/2 and 5/2
B. 1/2,-1/2 and -5/2
C. -1/2,1/2 and 3/2
D. 1/2, -1/2 and -3/2
![]() Q. 29 Four holes of radius R are cut from a thin square plate of side 4R and mass M. The moment of inertia of the remaining portion about z-axis is:
Q. 29 Four holes of radius R are cut from a thin square plate of side 4R and mass M. The moment of inertia of the remaining portion about z-axis is:
A. πMR²/12
B. (4/3 – π/4 ) MR²
C. (4/3 – π/6) MR²
D. (8/3 – 10π/16) MR²
![]() Q. 30 A liquid is kept in a cylindrical vessel which is being rotated about a vertical axis through the centre of the circular base. If the radius of the vessel is r and angular velocity of rotation is ω , then the difference in the heights of the liquid at the centre of the vessel and the edge is:
Q. 30 A liquid is kept in a cylindrical vessel which is being rotated about a vertical axis through the centre of the circular base. If the radius of the vessel is r and angular velocity of rotation is ω , then the difference in the heights of the liquid at the centre of the vessel and the edge is:
A. rω/2g
B. r²ω²/2g
C. √(2grω)
D. ω²/2gr²
![]() Q. 31 A block of mass 10 kg is moving in x-direction with a constant speed of 10m/s. It is subjected to a retarding force F = 0.1x joule/meter during its travel from x = 20 m to x = 30 m. Its final K.E will be :
Q. 31 A block of mass 10 kg is moving in x-direction with a constant speed of 10m/s. It is subjected to a retarding force F = 0.1x joule/meter during its travel from x = 20 m to x = 30 m. Its final K.E will be :
A. 475 J
B. 450 J
C. 275 J
D. 250 J
![]() Q. 32 A capillary tube of radius r is immersed in water and water rises in it to a height h. The mass of water in the capillary tube 5g. Another capillary tube of radius 2r is immersed in water. The mass of water that will rise in this tube is :
Q. 32 A capillary tube of radius r is immersed in water and water rises in it to a height h. The mass of water in the capillary tube 5g. Another capillary tube of radius 2r is immersed in water. The mass of water that will rise in this tube is :
A. 2.5 g
B. 5.0 g
C. 10 g
D. 20 g
![]() Q. 33 Which of the following pairs does not have same dimensions?
Q. 33 Which of the following pairs does not have same dimensions?
A. impulse and momentum
B. moment of inertia and moment of force
C. angular momentum and planck’s constant
D. work and torque
![]() Q. 34 The wave length of Lymen series for first number is
Q. 34 The wave length of Lymen series for first number is
A. (4 x 1.097 x 10⁷)m/3
B. 3m/(4×1.097×10⁷)
C. 4m/(3×1.097×10⁷ )
D. (3/4)m x 1.097 x 10⁷
![]() Q. 35 In the circuit shown, current flowing through 25 V cell is
Q. 35 In the circuit shown, current flowing through 25 V cell is
A. 7.2 A
B. 10 A
C. 12 A
D. 14.2 A
![]() Q. 36 Five sinusoidal waves have the same frequency 500Hz but their amplitudes are in the ratio 2 : 1/2 : 1/2 : 1: 1 and their phase angles 0,π/6,π/3,π/2, and respectively. The phase angle of resultant wave obtained by the super position of these five wave is:
Q. 36 Five sinusoidal waves have the same frequency 500Hz but their amplitudes are in the ratio 2 : 1/2 : 1/2 : 1: 1 and their phase angles 0,π/6,π/3,π/2, and respectively. The phase angle of resultant wave obtained by the super position of these five wave is:
A. 30⁰
B. 45⁰
C. 60⁰
D. 90⁰
![]() Q. 37 The second overtone of an open pipe has the same frequency as the first overtone of a closed pipe 2m long. The length of the open pipe is :
Q. 37 The second overtone of an open pipe has the same frequency as the first overtone of a closed pipe 2m long. The length of the open pipe is :
A. 8m
B. 4m
C. 2m
D. 1m
![]() Q. 38 Let T₁ and T₂ be the time periods of springs A and B mass M is suspended from the series combination, the time period is T, then
Q. 38 Let T₁ and T₂ be the time periods of springs A and B mass M is suspended from the series combination, the time period is T, then
A. T₁ + T₂ + T₃
B. 1/T = 1/T₁ + 1/T₂
C. T² = T₁² + T₂²
D. 1/T² = 1/T₁² + 1/T₂²
![]() Q. 39 Alternating current cannot be measured by D.C. ammeter because
Q. 39 Alternating current cannot be measured by D.C. ammeter because
A. A.C. cannot pass through D.C. ammeter
B. A.C. changes direction
C. average value of current for complete cycle is zero
D. D.C.ammeter will get damaged
![]() Q. 40 The core of any transformer is laminated so as to
Q. 40 The core of any transformer is laminated so as to
A. reduce the energy loss due to eddy currents
B. make it light weight
C. make it robust and strong
D. increase the secondary voltage
![]() Questions: 41 – 60
Questions: 41 – 60
Directions: In the following questions(41-60), a statement of assertion(A) is followed by a statement of a reason (R). Mark the correct choice is:
a. If both assertion and reason are true and reason is the correct explanation of assertion.
b. If both assertion and reason are true but reason is not the correct explanation of reason
c. If assertion is true but reason is false
d. If both assertion and reason are false.
![]() Q. 41 Assertion: Two balls of different masses are thrown vertically upward with same speed. They will pass though their point of projection in the downward direction with the same period.
Q. 41 Assertion: Two balls of different masses are thrown vertically upward with same speed. They will pass though their point of projection in the downward direction with the same period.
Reason: The maximum height and downward velocity attained at the point of projection are independent of the mass of the ball.
A. a
B. b
C. c
D. d
![]() Q. 42 Assertion: In javelin throw, the athlete throws the projectile at an angle slightly more than 45⁰ .
Q. 42 Assertion: In javelin throw, the athlete throws the projectile at an angle slightly more than 45⁰ .
Reason: The maximum range does not depend upon angle of projection.
A. a
B. b
C. c
D. d
![]() Q. 43 Assertion: The assertion weight of a body in an elevator moving with some downward acceleration is less than the actual weight weight of a body.
Q. 43 Assertion: The assertion weight of a body in an elevator moving with some downward acceleration is less than the actual weight weight of a body.
Reason: The part of the weight is spent in producing downward acceleration, when body is in elevator.
A. a
B. b
C. c
D. d
![]() Q. 44 Assertion: An electric field is preferred in comparison to magnetic field for detecting the electron beam in a television picture tube.
Q. 44 Assertion: An electric field is preferred in comparison to magnetic field for detecting the electron beam in a television picture tube.
Reason: Electric field requires low voltage.
A. a
B. b
C. c
D. d
![]() Q. 45 Assertion : A horse has to pull a cart harder during the first few steps of his motion.
Q. 45 Assertion : A horse has to pull a cart harder during the first few steps of his motion.
Reason: The first few steps are always difficult.
A. a
B. b
C. c
D. d
![]() Q. 46 Assertion : The magnetic poles of earth do not coincide with the geographic poles.
Q. 46 Assertion : The magnetic poles of earth do not coincide with the geographic poles.
Reason: The discrepancy between the orientation of a compass and the true north-south
direction is known as magnetic declination.
A. a
B. b
C. c
D. d
![]() Q. 47 Assertion: Electromagnetic waves are transverse in nature.
Q. 47 Assertion: Electromagnetic waves are transverse in nature.
Reason : The electric and magnetic fields of an e.m wave are perpendicular to each other and also perpendicular to the direction of wave propogation.
A. a
B. b
C. c
D. d
![]() Q. 48 Assertion: A wheel moving down a perfectly frictionless inclined plane will undergo slipping (not rolling motion)
Q. 48 Assertion: A wheel moving down a perfectly frictionless inclined plane will undergo slipping (not rolling motion)
Reason: For perfect rolling motion, work done against friction is zero.
A. a
B. b
C. c
D. d
![]() Q. 49 Assertion: A hollow shaft is found to be stronger than a solid shaft made of same material.
Q. 49 Assertion: A hollow shaft is found to be stronger than a solid shaft made of same material.
Reason : The torque required to produce a given twist in hollow cylinder is greater than
that required to twist a solid cylinder of same size and material.
A. a
B. b
C. c
D. d
![]() Q. 50 Assertion : Water kept in an open vessel will quickly evaporate on the surface of the moon.
Q. 50 Assertion : Water kept in an open vessel will quickly evaporate on the surface of the moon.
Reason : The temperature at the surface of the moon is much higher than boiling point of water.
A. a
B. b
C. c
D. d
![]() Q. 51 Assertion : A pure semiconductor has negative temperature coefficient of resistance.
Q. 51 Assertion : A pure semiconductor has negative temperature coefficient of resistance.
Reason : On raising the temperature, more charge carriers are released, conductance
increases and resistance decreases.
A. a
B. b
C. c
D. d
![]() Q. 52 Assertion: At a fixed temperature, silicon will have a minimum conductivity when it has smaller acceptor doping.
Q. 52 Assertion: At a fixed temperature, silicon will have a minimum conductivity when it has smaller acceptor doping.
Reason : The conductivity of an intrinsic semi conductor is slightly higher than that of a lightly doped p-type
A. a
B. b
C. c
D. d
![]() Q. 53 Assertion : communication in UHF/VHF regions can be established by space wave or troposhpheric wave.
Q. 53 Assertion : communication in UHF/VHF regions can be established by space wave or troposhpheric wave.
Reason: communication in UHF/VHF regions is limited to line of sight distance.
A. a
B. b
C. c
D. d
![]() Q. 54 Assertion : If objective and eye lenses of a microscope are interchanged then it can work as telescope.
Q. 54 Assertion : If objective and eye lenses of a microscope are interchanged then it can work as telescope.
Reason: The objective lens of telescope has small focal length.
A. a
B. b
C. c
D. d
![]() Q. 55 Assertion: If a proton and an alpha-particle enter a uniform magnetic field perpendicularly with a same speed the time period of revolution of alpha-particle is double that of proton.
Q. 55 Assertion: If a proton and an alpha-particle enter a uniform magnetic field perpendicularly with a same speed the time period of revolution of alpha-particle is double that of proton.
Reason: In a magnetic field, the period of revolution of a changed particle is directly proportional to the mass of the particle and is inversely proportional to change of particle.
A. a
B. b
C. c
D. d
![]() Q. 56 Assertion: If momentum of a body increases by 50% its kinetic energy will increase by 125%.
Q. 56 Assertion: If momentum of a body increases by 50% its kinetic energy will increase by 125%.
Reason: Kinetic energy is proportional to square of velocity.
A. a
B. b
C. c
D. d
![]() Q. 57 Assertion : The difference between in the value of acceleration due to gravity at pole and equator is proportional to square of angular velocity.
Q. 57 Assertion : The difference between in the value of acceleration due to gravity at pole and equator is proportional to square of angular velocity.
Reason : The value of acceleration due to gravity is minimum at the equator and maximum at the pole.
A. a
B. b
C. c
D. d
![]() Q. 58 Assertion : It is advantageous to transmit electriv power at high voltage.
Q. 58 Assertion : It is advantageous to transmit electriv power at high voltage.
Reason: High voltage implies high current.
A. a
B. b
C. c
D. d
![]() Q. 59 Assertion : X-ray astronomy is possible only from satellites orbiting the earth.
Q. 59 Assertion : X-ray astronomy is possible only from satellites orbiting the earth.
Reason : Efficiency of x-rays telescope is large as compared to any other telescope.
A. a
B. b
C. c
D. d
![]() Q. 60 Assertion : The de broglie equation has significance for any microscopic or submicroscopic particles.
Q. 60 Assertion : The de broglie equation has significance for any microscopic or submicroscopic particles.
Reason : The de broglie wave lengths inversely proportional to the mass of the object if velocity is constant.
A. a
B. b
C. c
D. d
![]() Q. 61 Butter is an example of which type of colloid?
Q. 61 Butter is an example of which type of colloid?
A. solid in liquid
B. liquid in solid
C. liquid in liquid
D. gas in liquid
![]() Q. 62 What are constituents of misch metal?
Q. 62 What are constituents of misch metal?
A. La,Fe
B. La,Ce
C. Fe,Ce
D. Ce,Cu
![]() Q. 63 For a 1st order reaction if concentration is doubled then rate of reaction becomes:
Q. 63 For a 1st order reaction if concentration is doubled then rate of reaction becomes:
A. doubles
B. half
C. four times
D. remains same
![]() Q. 64 In tetragonal crystal system,which of the following is not true?
Q. 64 In tetragonal crystal system,which of the following is not true?
A. All axial lengths and all axial angles are equal
B. all three axial length are equal
C. all three axial angles are equal
D. two axial angles are equal but the third is different
![]() Q. 65 Which of the following is correct?
Q. 65 Which of the following is correct?
A. ionic radius is proportional to atomic number
B. ionic radius is inversely proportional to atomic mass
C. Ionic radius is inversely proportional to effective nuclear charge
D. all are correct
![]() Q. 66 The strained tetracyclic alkane is isomerize thermally to the cyclic alkene. The reaction involves:
Q. 66 The strained tetracyclic alkane is isomerize thermally to the cyclic alkene. The reaction involves:
A. free radical
B. carbocation
C. carbanion
D. carbene
![]() Q. 67 The product is:
Q. 67 The product is:
A. a
B. b
C. c
D. d
![]() Q. 68 For a reaction X → Y , the graph of the product concentration (x) versus time (t) came out to be a straight line passing through the origin. Hence the graph of -d[X]/dt and time would be:
Q. 68 For a reaction X → Y , the graph of the product concentration (x) versus time (t) came out to be a straight line passing through the origin. Hence the graph of -d[X]/dt and time would be:
A. straight line with a negative slope and intercept on y-axis
B. straight line with a positive slope and an intercept on y-axis
C. a straight line parallel to x-axis
D. a hyperbola
![]() Q. 69 A factory produces 40 kg of calcium in two hours by electrolysis. How much a aluminium can be produced by same current in 2 hours if current efficiency is 50% ?
Q. 69 A factory produces 40 kg of calcium in two hours by electrolysis. How much a aluminium can be produced by same current in 2 hours if current efficiency is 50% ?
A. 22 kg
B. 18 kg
C. 9 kg
D. 27 kg
![]() Q. 70 Equal weight of CO and CH₄ are mixed together in an empty container at 300K. The fraction of total pressure exerted by CH₄ is
Q. 70 Equal weight of CO and CH₄ are mixed together in an empty container at 300K. The fraction of total pressure exerted by CH₄ is
A. 16/17
B. 7/11
C. 8/9
D. 5/16
![]() Q. 71 Match list I with list II and select the correct answer using the codes given below the lists.
Q. 71 Match list I with list II and select the correct answer using the codes given below the lists.
A. A-1,B-3,C-5,D-4
B. A-2,B-3,C-5,D-1
C. A-4,B-3,C-5,D-1
D. A-4,B-5,C-3,D-1
![]() Q. 72 which of the following reactions does not yield an amine?
Q. 72 which of the following reactions does not yield an amine?
A. a
B. b
C. c
D. d
![]() Q. 73 The chemical name for melamine is :
Q. 73 The chemical name for melamine is :
A. 1,3,5-Triamino-2,4,6-triazine
B. 2,4,6-Triamino-1,3,5-triazine
C. 2-Amino-1,3,5-triazine
D. 2,4-Diamino-1,3,5-triazine
![]() Q. 74 Bromine is added to cold the dilute aqueous solution of NaOH. The mixture is boiled. Which of the following statements is not true?
Q. 74 Bromine is added to cold the dilute aqueous solution of NaOH. The mixture is boiled. Which of the following statements is not true?
A. During the reaction bromine is present in four different oxidation states
B. The greatest difference between the various oxidation states of bromine is 5
C. on acidification of the final mixture bromine is formed
D. disproportionation of bromine occurs during the reaction
![]() Q. 75 A complex PtCl₄ 5NH₃ shows a molar conductance of 402 ohm⁻¹ cm² mol⁻¹ in water and precipitates three moles of AgCl with AgNO₃ solution. The formula of the complex is
Q. 75 A complex PtCl₄ 5NH₃ shows a molar conductance of 402 ohm⁻¹ cm² mol⁻¹ in water and precipitates three moles of AgCl with AgNO₃ solution. The formula of the complex is
A. [pt(NH₃)₆]Cl₄
B. [pt(NH₃)₄Cl₂]Cl₂
C. [Pt(NH₃)₅Cl]Cl₃
D. [Pt(NH₃)₃Cl₃]Cl
![]() Q. 76 using appropriate molar conductances of the electrolytes listed above at infinite dilution in H₂O at 25⁰ C
Q. 76 using appropriate molar conductances of the electrolytes listed above at infinite dilution in H₂O at 25⁰ C
A. 517.2
B. 552.7
C. 390.7
D. 217.5
![]() Q. 77 In the ground state of Cu, the number of shells occupied, sub shells occupied, filled orbitals and unpaired electrons respectively are:
Q. 77 In the ground state of Cu, the number of shells occupied, sub shells occupied, filled orbitals and unpaired electrons respectively are:
A. 4,8,15,0
B. 3,6,15,1
C. 3,6,14,0
D. 4,7,14,2
![]() Q. 78 Which of the following conditions is not correct for reasoning structures?
Q. 78 Which of the following conditions is not correct for reasoning structures?
A. the contributing structures must have the same number unpaired electrons
B. the contributing structures should have similar energies
C. the contributing structures should be so written that unlike charges reside on atoms that are far apart
D. The positive charge should be present on the electro positive element and the negative charge on the electronegative element
![]() Q. 79 CaO and NaCl have the same crystal structure and approximately the same ionic radii. If U is lattice energy of Nacl, the appropriate lattice energy of CaO is:
Q. 79 CaO and NaCl have the same crystal structure and approximately the same ionic radii. If U is lattice energy of Nacl, the appropriate lattice energy of CaO is:
A. U/2
B. U
C. 2U
D. 4U
![]() Q. 80 The phosphate of a metal has the formula MHPO₄. The formula of its chloride would be:
Q. 80 The phosphate of a metal has the formula MHPO₄. The formula of its chloride would be:
A. MCl
B. MCl₂
C. MCl₃
D. M₂Cl₃
![]() Q. 81 Two flasks X and Y have capacity 1L and 2L respectively and each of them contains 1 mole of a gas. The temperature of the flasks are so adjusted that average speed of molecules in X is twice as those in Y.The pressure in flask X would be:
Q. 81 Two flasks X and Y have capacity 1L and 2L respectively and each of them contains 1 mole of a gas. The temperature of the flasks are so adjusted that average speed of molecules in X is twice as those in Y.The pressure in flask X would be:
A. same as that Y
B. half of that in Y
C. twice of that in Y
D. 8 times of that Y
![]() Q. 82 Match the list I and list II and select the correct answer using the codes given below the lists:
Q. 82 Match the list I and list II and select the correct answer using the codes given below the lists:
A. A-5,B-1,C-2,D-4
B. A-5,B-3,C-2,D-4
C. A-3,B-5,C-2,D-1
D. A-5,B-3,C-2,D-1
![]() Q. 83 What of the following sequence contains atomic number of only representative elements?
Q. 83 What of the following sequence contains atomic number of only representative elements?
A. 55,12,48,53
B. 13,33,54,80
C. 3,33,53,87
D. 22,33,55,66
![]() Q. 84 Which of the following contains atomic number of only representative elements?
Q. 84 Which of the following contains atomic number of only representative elements?
A. 55, 12, 48, 53
B. 13, 33, 54, 80
C. 3, 33, 53, 87
D. 22, 33, 55, 66
![]() Q. 85 100 cm³ of a given sample of H₂O₂ gives 1000 cm³ of O₂ at S.T.P. The given sample is :
Q. 85 100 cm³ of a given sample of H₂O₂ gives 1000 cm³ of O₂ at S.T.P. The given sample is :
A. 10% H₂O₂
B. 90% H₂O₂
C. 10 volume H₂O₂
D. 100 volume H₂O₂
![]() Q. 86 Beryllium and aluminium exhibit many properties which are similar . But the two elements differ in:
Q. 86 Beryllium and aluminium exhibit many properties which are similar . But the two elements differ in:
A. maximum covalency in compounds
B. exhibiting atmospheric nature in their oxides
C. forming covalent halides
D. forming polymeric hydrides
![]() Q. 87 Cyclohexene on ozonolysis followed by reaction with zinc dust and water gives compound E. Compound E further treatment with aqueous KOH yields compound F. Compound F is
Q. 87 Cyclohexene on ozonolysis followed by reaction with zinc dust and water gives compound E. Compound E further treatment with aqueous KOH yields compound F. Compound F is
A. a
B. b
C. c
D. d
![]() Q. 88 The product P will be:
Q. 88 The product P will be:
A. a
B. b
C. c
D. d
![]() Q. 89 The compound which on reaction with aqueous nitrous acid at low temperature produces an oily nitrosoamine is:
Q. 89 The compound which on reaction with aqueous nitrous acid at low temperature produces an oily nitrosoamine is:
A. methyl amine
B. ethyl amine
C. diethyl amine
D. triethyl amine
![]() Q. 90 Compound A (molecular formula C₃H₈O) is treated with acidified potassium dichromate to form a product B(molecular formula C₃H₆O). B forms a shining silver mirror on warming with ammoniacal silver nitrate. B when treated with an aqueous solution of H₂NCONHNH₂. HCl and sodium acetate gives a product C. Identify the structure of C.
Q. 90 Compound A (molecular formula C₃H₈O) is treated with acidified potassium dichromate to form a product B(molecular formula C₃H₆O). B forms a shining silver mirror on warming with ammoniacal silver nitrate. B when treated with an aqueous solution of H₂NCONHNH₂. HCl and sodium acetate gives a product C. Identify the structure of C.
A. a
B. b
C. c
D. d
![]() Q. 91 Assume that you are travelling at speed of 90km/h in a small car with a mass of 1050 kg. If the uncertainty in the velocity of the car is 1%(Δv=0.9 km/h), what is the uncertainty (in meters) in the position of the car?
Q. 91 Assume that you are travelling at speed of 90km/h in a small car with a mass of 1050 kg. If the uncertainty in the velocity of the car is 1%(Δv=0.9 km/h), what is the uncertainty (in meters) in the position of the car?
A. Δx ≥1 x 10⁻³⁵m
B. Δx ≥2 x 10⁻³⁷m
C. Δx ≥2 x 10⁻³⁶m
D. Δx≥4 x 10⁻³m
![]() Q. 92 When 25g of Na₂SO₄ is dissolved in 10³ kg of solution, its concentration will be:
Q. 92 When 25g of Na₂SO₄ is dissolved in 10³ kg of solution, its concentration will be:
A. 2.5 ppm
B. 25 ppm
C. 250 ppm
D. 100 ppm
![]() Q. 93 Degree of unsaturation of A =2 , it contains no double or triple bonds.
Q. 93 Degree of unsaturation of A =2 , it contains no double or triple bonds.
A. a
B. b
C. c
D. d
![]() Q. 94 The shape and hybridisation of some xenon oxyfluorides are given. Choose the wrong set.
Q. 94 The shape and hybridisation of some xenon oxyfluorides are given. Choose the wrong set.
A. XeOF₂- T -shape-sp³d
B. XeOF₄-square pyramidal-sp³d²
C. XeO₂F₂-Distorted trigonal bipyramidal-sp³d
D. XeO₃F₂-Octahedral-sp³d
![]() Q. 95 The standard half cell reduction potential for Ag’ I Ag is 0.7991 V at 25⁰ C . Given the experimental value K = 1.56 x 10 for AgCl, calculate the standard half cell potential for the Ag IAgCl electrode.
Q. 95 The standard half cell reduction potential for Ag’ I Ag is 0.7991 V at 25⁰ C . Given the experimental value K = 1.56 x 10 for AgCl, calculate the standard half cell potential for the Ag IAgCl electrode.
A. 0.2192 V
B. -0.2192 V
C. -1.2192 V
D. 1.2192 V
![]() Q. 96 Which of the following acids will not evolve H₂ gas on reaction with alkali metals?
Q. 96 Which of the following acids will not evolve H₂ gas on reaction with alkali metals?
A. hydrazoic acids
B. perxenic acid
C. boric acid
D. none of these
![]() Q. 97 The major product of the following reaction is:
Q. 97 The major product of the following reaction is:
A. a
B. b
C. c
D. d
![]() Q. 98 Stomach acid, a dilute solution of HCl in water, can be neutralized by reaction with sodium hydrogen carbonate,how many millimeters of 0.125 M NaHCO₃ solution are needed to neutralize 18.0mL of 0.100 M HCl?
Q. 98 Stomach acid, a dilute solution of HCl in water, can be neutralized by reaction with sodium hydrogen carbonate,how many millimeters of 0.125 M NaHCO₃ solution are needed to neutralize 18.0mL of 0.100 M HCl?
A. 14.4mL
B. 12.0mL
C. 14.0mL
D. 13.2mL
![]() Q. 99 For the electrochemical cell, M I M⁺ II X⁻ I X , E(M⁺ I M) = 0.44 V and E⁰(X I X⁻) = 0.33 V. From this data one can deduce that
Q. 99 For the electrochemical cell, M I M⁺ II X⁻ I X , E(M⁺ I M) = 0.44 V and E⁰(X I X⁻) = 0.33 V. From this data one can deduce that
A. a
B. b
C. c
D. d
![]() Q. 100 Which is optically inactive?
Q. 100 Which is optically inactive?
A. a
B. b
C. c
D. d
![]() Questions: 101 – 120
Questions: 101 – 120
Directions:In the following (101-120), a statement of assertion (A) is followed by a statement of a reason(R). Mark the correct choice as :
(a) If both assertion and reason are true and reason is the correct explanation of the assertion.
(b) If the assertion and reason are true but reason is not the correct explanation of assertion
(c) If assertion is true but reason is false
(d) If both assertion and reason are false
![]() Q. 101 Assertion : Magnesium is extracted by the electrolysis of fused mixture of MgCl₂ NaCl and CaCl₂.
Q. 101 Assertion : Magnesium is extracted by the electrolysis of fused mixture of MgCl₂ NaCl and CaCl₂.
Reason : calcium chloride acts as a reducing agent.
A. a
B. b
C. c
D. d
![]() Q. 102 Assertion : The equilibrium constant is fixed and a characteristic any given chemical reaction at a specified temperature.
Q. 102 Assertion : The equilibrium constant is fixed and a characteristic any given chemical reaction at a specified temperature.
Reason : The composition of the final equilibrium mixture at a particular temperature
depends upon the starting amount of reactants.
A. a
B. b
C. c
D. d
![]() Q. 103 Assertion : PCl₅ is covalent in gaseous and liquid states but ionic in solid state. Reason : PCl₅ in solid state consists of tetrahedral PCl₄⁺ cation and octahedral PCl₆⁻ anion.
Q. 103 Assertion : PCl₅ is covalent in gaseous and liquid states but ionic in solid state. Reason : PCl₅ in solid state consists of tetrahedral PCl₄⁺ cation and octahedral PCl₆⁻ anion.
A. a
B. b
C. c
D. d
![]() Q. 104 Assertion : Zinc displaces copper from copper sulphate solution.
Q. 104 Assertion : Zinc displaces copper from copper sulphate solution.
Reason : The E⁰ of zinc is -0.76 V and that of copper is + 0.34 V.
A. a
B. b
C. c
D. d
![]() Q. 105 Reason : Principle functional group gets lowest number followed by double bond or triple bond.
Q. 105 Reason : Principle functional group gets lowest number followed by double bond or triple bond.
A. a
B. b
C. c
D. d
![]() Q. 106 Assertion : Helium has the highest value of ionisation energy among all the elements known.
Q. 106 Assertion : Helium has the highest value of ionisation energy among all the elements known.
Reason : Helium has the highest value of ionisation energy among all the elements known.
A. a
B. b
C. c
D. d
![]() Q. 107 Assertion : The nuclear isomers are the atoms with the same atomic number and same mass number , but with different radioactive properties.
Q. 107 Assertion : The nuclear isomers are the atoms with the same atomic number and same mass number , but with different radioactive properties.
Reason : The nucleus in the excited state will evidently have a different half-life as compared to that in a ground state.
A. a
B. b
C. c
D. d
![]() Q. 108 Assertion : Conductivity of silicon increases by doping it with group -15 elements.
Q. 108 Assertion : Conductivity of silicon increases by doping it with group -15 elements.
Reason : Doping means introduction of small amount of impurities like P, As or Bi into the pure crystals.
A. a
B. b
C. c
D. d
![]() Q. 109 Assertion : The overall order of the reaction is the sum of the exponents of all the reactants in the rate expressions.
Q. 109 Assertion : The overall order of the reaction is the sum of the exponents of all the reactants in the rate expressions.
Reason : There are many higher order reactions.
A. a
B. b
C. c
D. d
![]() Q. 110 Assertion : Transition metals are poor reducing agents.
Q. 110 Assertion : Transition metals are poor reducing agents.
Reason : Transition metals form numerous alloys with other metals.
A. a
B. b
C. c
D. d
![]() Q. 111 Assertion : Aldol condensation can be catalysed both by acids and bases.
Q. 111 Assertion : Aldol condensation can be catalysed both by acids and bases.
Reason : β-hydroxy aldehydes or ketones readily undergo acid catalysed dehydration.
A. a
B. b
C. c
D. d
![]() Q. 112 Assertion : The position of an element in periodic table after emission of the one α- and two β-particles remains unchanged.
Q. 112 Assertion : The position of an element in periodic table after emission of the one α- and two β-particles remains unchanged.
Reason : Emission of one α- and two β-particles give isotope of the element which acquires same position in periodic table.
A. a
B. b
C. c
D. d
![]() Q. 113 Assertion : S.I unit of atomic mass and molecular mass in kilograms.
Q. 113 Assertion : S.I unit of atomic mass and molecular mass in kilograms.
Reason : Atomic mass is equal to the mass of 6.023 x 10²⁴ atoms.
A. a
B. b
C. c
D. d
![]() Q. 114 Assertion : Bond energy and bond dissociation energy have identical value for diatomic molecules.
Q. 114 Assertion : Bond energy and bond dissociation energy have identical value for diatomic molecules.
Reason : Greater the bond dissociation energy, less reactive is the bond.
A. a
B. b
C. c
D. d
![]() Q. 115 Assertion : The degree of complex formation in actinides decreases in the order M⁺⁴ > MO₂⁺² > M⁺³ > MO₂⁺
Q. 115 Assertion : The degree of complex formation in actinides decreases in the order M⁺⁴ > MO₂⁺² > M⁺³ > MO₂⁺
Reason : Actinides form complexes with π- bonding ligands such as alkyl phosphines and thioethers.
A. a
B. b
C. c
D. d
![]() Q. 116 Assertion : Benzene on heating with conc.HSO gives benzene sulphonic acid which when heated with superheated steam under pressure gives benzene.
Q. 116 Assertion : Benzene on heating with conc.HSO gives benzene sulphonic acid which when heated with superheated steam under pressure gives benzene.
Reason : Sulphonation is a reversible process.
A. a
B. b
C. c
D. d
![]() Q. 117 Assertion : The molarity of the solution does not change with change in temperature.
Q. 117 Assertion : The molarity of the solution does not change with change in temperature.
Reason : The molarity is expressed in units of moles per 1000 g of solvent.
A. a
B. b
C. c
D. d
![]() Q. 118 Assertion : Due to Frenkel defect, density of the crystalline solid decreases.
Q. 118 Assertion : Due to Frenkel defect, density of the crystalline solid decreases.
Reason : In Frenkel defect, cation or anion leaves the crystal.
A. a
B. b
C. c
D. d
![]() Q. 119 Assertion : is named as tetrakis (ethylene-diammine) μ- hydroxo-μ -midodicobalt(III) ion.
Q. 119 Assertion : is named as tetrakis (ethylene-diammine) μ- hydroxo-μ -midodicobalt(III) ion.
Reason : In naming polynulear complexes i.e.,containing two or more metal atoms joined by briging ligands, the word μ is added with hyphen before the name of such ligands.
A. a
B. b
C. c
D. d
![]() Q. 120 Assertion : 2,3 – Dimethyl but-2-ene is more stable than but-2-ene.
Q. 120 Assertion : 2,3 – Dimethyl but-2-ene is more stable than but-2-ene.
Reason : Six hyper conjugation structures can be written for 2,3-dimethyl but-2-ene while but-2-ene has twelve.
A. a
B. b
C. c
D. d
![]() Q. 121 Vitamin B₆ is also called as
Q. 121 Vitamin B₆ is also called as
A. thiamine
B. pantothenic acid
C. pyridoxine
D. retinol
![]() Q. 122 Protista differs monera in having
Q. 122 Protista differs monera in having
A. cell wall
B. autotrophic nutrition
C. flagella
D. nuclear membrane
![]() Q. 123 What does ‘T’ stands for in DTP for in DTP vaccine?
Q. 123 What does ‘T’ stands for in DTP for in DTP vaccine?
A. tuberculosis
B. autotrophic nutrtion
C. trachoma
D. tetanus
![]() Q. 124 Why are vascular bundles closed in monocots?
Q. 124 Why are vascular bundles closed in monocots?
A. xylem and phloem are present
B. xylem and phloem occur in separate bundles
C. vascular and cambium is separate between xylem and phloem
D. vascular cambium is not present
![]() Q. 125 Who invented electron microscope?
Q. 125 Who invented electron microscope?
A. Janssen
B. edison
C. knoll and ruska
D. landsteiner
![]() Q. 126 What do A,B,C,D represent in the following figure?
Q. 126 What do A,B,C,D represent in the following figure?
A. A: carrier protein, B : symport, C : uniport, D : antiport
B. A: carrier protein,B: uniport,C: antiport,D:symport
C. A:carrier protein,B:antiport,C:symport,D:uniport
D. A:carrier protein,B:uniport, C: symport,D:antiport
![]() Q. 127 Gametophyte and sporophyte are independent of each other in which of the following groups?
Q. 127 Gametophyte and sporophyte are independent of each other in which of the following groups?
A. pteridophytes
B. anangiosperms
C. gymnosperms
D. bryophytes
![]() Q. 128 Which of the following is correct?
Q. 128 Which of the following is correct?
A. paneth cells secrete pepsiniogen
B. parietal cells secrete hydrochloric acid
C. argentaffin cells secrete mucus
D. chief cells secrete gastrin
![]() Q. 129 Which of the following has highest diversity in India?
Q. 129 Which of the following has highest diversity in India?
A. mango
B. dolphin
C. tiger
D. orchids
![]() Q. 130 Which of the following is correct about the given figure?
Q. 130 Which of the following is correct about the given figure?
A. the length of the thick and thin myofilaments has changes
B. length of both anisotropic and isotropic band has changed
C. the myosin cross-bridges move on the surface of actin and the thin and thick myofilaments slide past each other
D. length of the sarcomere remains same
![]() Q. 131 Which of the following disorders are caused due to recessive autosomal mutations?
Q. 131 Which of the following disorders are caused due to recessive autosomal mutations?
A. turners’s syndrome and sickle cell anaemia
B. Edward’s syndrome and Down’s syndrome
C. cystic fibrosis and phenylketonuria
D. Alzheimer’s disease and Huntington’s chorea
![]() Q. 132 What is correct about the movement of substance across the membrane in facilitated diffusion?
Q. 132 What is correct about the movement of substance across the membrane in facilitated diffusion?
A. it is an active transport
B. it doesn’t cause transport of molecules from low concentration to high concentration
C. it is insensitive to inhibitors
D. it is a very specific transport
![]() Q. 133 Which one is correct?
Q. 133 Which one is correct?
A. salmonella typhi and haemophilus influence cause pneumonia
B. widal test is done for malaria
C. entamoea histolytica causes amoebiasis
D. wucheria causes enterobiasis
![]() Q. 134 What is greek word for ecology?
Q. 134 What is greek word for ecology?
A. ethology
B. oekologie
C. synecology
D. hexicology
![]() Q. 135 Which of the following is correct regarding genetic code?
Q. 135 Which of the following is correct regarding genetic code?
A. UUU is the initiation codon which also codes for phenylalanine
B. there are 64 triplet codons and only 20 amino acids
C. three random nitrogen bases specify the placement of one amino acid
D. UAA is the non sense codon which also codes methionine
![]() Q. 136 The given figure shows L.S of the seed of maize, What do A,B,C and D represent?
Q. 136 The given figure shows L.S of the seed of maize, What do A,B,C and D represent?
A. A:endosperm, B:scutellum, C:plumule,D:coleoptile
B. A:scutellum,B:pericarp,C:radicle,D:coleoptile
C. A:endosperm,B:scutellum,C:radicle,D:coleorrhiza
D. A:scutellum,B:pericarp,C:plumule,D:coleorrhiza
![]() Q. 137 Refer the given figures on photoperiodism and select the correct opinion:
Q. 137 Refer the given figures on photoperiodism and select the correct opinion:
A. A-no correlation between light period and flowering B-long light exposure period Cshort light exposure period
B. A-long light exposure period, B-no correlation between light period and flowering Cshort light exposure period
C. A-short light exposure period, B-long light exposure, C-no correlation between light period and flowering
D. A-no correlation between exposure light period and flowering,B-short light exposure period,C-long light exposure period
![]() Q. 138 What are singer and nicolson known for?
Q. 138 What are singer and nicolson known for?
A. one-gene-one-enzyme hypothesis
B. plasma membrane modifications
C. fluid-mosaic model of plasma membrane
D. structure of DNA
![]() Q. 139 Select the correct statement.
Q. 139 Select the correct statement.
A. Acetobacter aceti produces citric acid
B. Saccharomyces cerevisiae is used as clot buster
C. penicillium notatum restrict thje growth of staphylococci
D. methanogens are found in aerobic conditions
![]() Q. 140 Which of the following disease is also called chritistmas disease?
Q. 140 Which of the following disease is also called chritistmas disease?
A. sickle-cell anaemia
B. haemoglobinunuria
C. myocardial infarction
D. haemophilia -B
![]() Q. 141 Which of the following is correct ?
Q. 141 Which of the following is correct ?
A. all fungi are filamentous
B. transfer of DNA from one bacteria to another bacteria cannot take place
C. Virus cannot have both DNA and RNA
D. protists reproduce asexually only
![]() Q. 142 Which of the following have porous body and are diploblastic?
Q. 142 Which of the following have porous body and are diploblastic?
A. Aurelia and obelia
B. Adamsia and Euplectella
C. lenucosolenia and spongilla
D. sycon and hydra
![]() Q. 143 CD-4 receptor is associated with
Q. 143 CD-4 receptor is associated with
A. AIDS
B. cancer
C. malaria
D. pneumonia
![]() Q. 144 Which one is orrect regarding electrocardigraph (ECG)?
Q. 144 Which one is orrect regarding electrocardigraph (ECG)?
A. P-wave represents the electrical excitation of the ventricle
B. QRS complex represents repolarisation of the ventricles
C. T-wave represnts repolarisation of the atria
D. by counting the number of QRS complexes one can determine the pulse rate
![]() Q. 145 Animals take phosphorous from
Q. 145 Animals take phosphorous from
A. water
B. plants
C. rock
D. soil
![]() Q. 146 What is the effect of GnRH produced by hypothalamus?
Q. 146 What is the effect of GnRH produced by hypothalamus?
A. stimulate the synthesis asnd secretion of androgens
B. stimulates secretion of milk in mammary glands
C. stimulate foetal ejection reflex
D. stimulates synthesis of carbohydrates from non-carbohydrates in liver
![]() Q. 147 Chemosensitive area of respiratory centre in medulla is affected by:
Q. 147 Chemosensitive area of respiratory centre in medulla is affected by:
A. less CO₂ and H⁺ ions
B. less O₂ and H⁺ ions
C. excess CO₂ and H⁺ ions
D. excess O₂ and H⁺ ions
![]() Q. 148 what do A,B,C and D represent?
Q. 148 what do A,B,C and D represent?
A. A-infundibulum,B-fertilization,C-myometrium,D-morula
B. A-infundibulum,B-fertilization,C-myometrium,D-blastocyst
C. A-isthmus,B-fertilization,C-myometrium,D-blastocyst
D. A-isthmus,B-fertilization,C-endometrium,D-morula
![]() Q. 149 Microvilli of intestinal epithelium are similar in function with:
Q. 149 Microvilli of intestinal epithelium are similar in function with:
A. typhlosole in earthworm
B. hepatic caecae in cockroach
C. intenstinal caecum in earth worm
D. malpighian tubules in cockroach
![]() Q. 150 The type of epithelial cells which line the inner surface of fallopian tubes, bronchioles and bronchi are known as:
Q. 150 The type of epithelial cells which line the inner surface of fallopian tubes, bronchioles and bronchi are known as:
A. squamous epithelium
B. ciliated epithelium
C. columnar epithelium
D. cubical epithelium
![]() Q. 151 cyclic photo phosphorylation involves:
Q. 151 cyclic photo phosphorylation involves:
A. PS I
B. PS II
C. PS I and PS II
D. PS₆₈ᵤ
![]() Q. 152 Which animal has the longest gestation period?
Q. 152 Which animal has the longest gestation period?
A. shark
B. walrus
C. elephant
D. dog
![]() Q. 153 What is a plasmid?
Q. 153 What is a plasmid?
A. bacterial, linear,dsDNA
B. extrachromosomal linear RNA
C. ectrachromosomal circular dsDNA
D. autonomously replicating circular RNA
![]() Q. 154 The concept of chemical evolution is based on
Q. 154 The concept of chemical evolution is based on
A. interaction of water,air and clay under intense heat
B. effect of solar radiation on chemicals
C. possible origin of life by combination of chemicals under suitable environmental conditions
D. crystallization of chemicals
![]() Q. 155 Which of the following is a correct match between crop, variety and resistance to diseases?
Q. 155 Which of the following is a correct match between crop, variety and resistance to diseases?
A. crop-wheat,variety-himgiri,resistance to diseases-white rust
B. crop-brassica,variety-pusa sadabahar,resistance to disease-black rot
C. crop-cowpea,variety-pusa komal, resistance to diseases-bacterial blight
D. crop-chilli,variety-pusa swarnim, resistance to diseases-chilly mosaic virus
![]() Q. 156 Recombinant DNA technology involves several steps in which initial step is of isolation of the DNA.Which enzymes are used in the process for the vreak down of fungal cell,plant cell and bacterial cell respectively?
Q. 156 Recombinant DNA technology involves several steps in which initial step is of isolation of the DNA.Which enzymes are used in the process for the vreak down of fungal cell,plant cell and bacterial cell respectively?
A. lysozyme, lipases, trypsin
B. chitinase, cellulase, lysozyme
C. chitinase, cellulase, trypsin
D. tripsin, lipases, cellulase
![]() Q. 157 The taxon which includes related species is
Q. 157 The taxon which includes related species is
A. class
B. order
C. family
D. genus
![]() Q. 158 Match the following columns and select the correct option:
Q. 158 Match the following columns and select the correct option:
A. A-(ii), B-(v),C-(i),D-(iii),E-(iv)
B. A-(iv), B-(i),C-(v),D-(iii),E-(ii)
C. A-(ii), B-(v),C-(iii),D-(i),E-(iv)
D. A-(iv), B-(i),C-(v),D-(ii),E-(iii)
![]() Q. 159 Which of the following is correct regarding HIV, hepatitis B, gonorrhoea, trichomoniasis?
Q. 159 Which of the following is correct regarding HIV, hepatitis B, gonorrhoea, trichomoniasis?
A. trichomoniasis is a STD whereas other are not
B. gonorrhoea is a viral disease whereas others are bacterial
C. HIV is a pathogen whereas others are diseases
D. Hepatitis B is eradicated completely whereas others are not
![]() Q. 160 The first stable product of calvin cycle is
Q. 160 The first stable product of calvin cycle is
A. 3-phopsphoglycerate
B. 1,3 biphosphoglycerate
C. glyceraldehyde-3 phosphate
D. ribulose – 5 – phosphate
![]() Questions: 161 – 180
Questions: 161 – 180
Directions: In the following questions(161-180),a statement of assertion(A) is followed by a statement of reason (R). Mark the correct choice as:
(a) If both assertion and reason are true and reason is the correct explanation of assertion
(b) If both assertion and reason are true but reason is not the correct explanation of assertion
(c) if assertion is true but reason is false
(d)if both assertion and reason are false
![]() Q. 161 Assertion : Adernaline is called an emergency harmone.
Q. 161 Assertion : Adernaline is called an emergency harmone.
Reason: It acts on the cells of skeletal, cardiac, smooth muscles, blood vessels and fat cells.
A. a
B. b
C. c
D. d
![]() Q. 162 Assertion : cork prevents the loss of water by evaporation.
Q. 162 Assertion : cork prevents the loss of water by evaporation.
Reason : cork cells contain tannins.
A. a
B. b
C. c
D. d
![]() Q. 163 Assertion : In cockroach respiratory gases directly comes in contact with the various organs of the body.
Q. 163 Assertion : In cockroach respiratory gases directly comes in contact with the various organs of the body.
Reason : Cockroaches do not have respiratory pigment
A. a
B. b
C. c
D. d
![]() Q. 164 Assertion : interferons are antiviral proteins.
Q. 164 Assertion : interferons are antiviral proteins.
Reason : Interferons are secreted by virus infected cells.
A. a
B. b
C. c
D. d
![]() Q. 165 Assertion : while on going down the loop of henle, the filtrate becomes hypotonic.
Q. 165 Assertion : while on going down the loop of henle, the filtrate becomes hypotonic.
Reason : The descending limb of loop of henle is impermeable to both water and electrolytes.
A. a
B. b
C. c
D. d
![]() Q. 166 Assertion: Shrinkage of the protoplast of a cell occurs under the influence of hypertonic solution.
Q. 166 Assertion: Shrinkage of the protoplast of a cell occurs under the influence of hypertonic solution.
Reason : Hypertonic solution causes plasmolysis.
A. a
B. b
C. c
D. d
![]() Q. 167 Assertion : In breeding produces pureline.
Q. 167 Assertion : In breeding produces pureline.
Reason : It causes homozygosity.
A. a
B. b
C. c
D. d
![]() Q. 168 Assertion : parturition is induced by neural signal in meternal pituitary.
Q. 168 Assertion : parturition is induced by neural signal in meternal pituitary.
Reason: At the end of gestation period, the maternal pituitary release prolactin which causes uterine contractions.
A. a
B. b
C. c
D. d
![]() Q. 169 Assertion : Commelina shows cleistogamy.
Q. 169 Assertion : Commelina shows cleistogamy.
Reason : This reduces chances of inbreeding.
A. a
B. b
C. c
D. d
![]() Q. 170 Assertion : antirrhinum is a good example to understand incomplete dominance.
Q. 170 Assertion : antirrhinum is a good example to understand incomplete dominance.
Reason : Heterozygotes show characteristics of both the alleles.
A. a
B. b
C. c
D. d
![]() Q. 171 Assertion : presence of large amounts of nutrients in water body causes excessive growth of planktonic algae.
Q. 171 Assertion : presence of large amounts of nutrients in water body causes excessive growth of planktonic algae.
Reason : It is due to planktonic algae.
A. a
B. b
C. c
D. d
![]() Q. 172 Assertion : bile is essential for the digestion of lipids.
Q. 172 Assertion : bile is essential for the digestion of lipids.
Reason : bile juice contains enzymes bilirubin and biliverdin.
A. a
B. b
C. c
D. d
![]() Q. 173 Assertion : emphysema is a chronic disorder in which alveolar walls are damaged.
Q. 173 Assertion : emphysema is a chronic disorder in which alveolar walls are damaged.
Reason : emphysema is closely related to cigaretta smoking.
A. a
B. b
C. c
D. d
![]() Q. 174 Assertion : DNA finger printing involves identifying differences in specific regions in DNA sequence.
Q. 174 Assertion : DNA finger printing involves identifying differences in specific regions in DNA sequence.
Reason : DNA fingerprinting is the basis of peternity testing.
A. a
B. b
C. c
D. d
![]() Q. 175 Assertion : all pathogens are parasites but all parasites are not pathogens.
Q. 175 Assertion : all pathogens are parasites but all parasites are not pathogens.
Reason : majority of the parasites confer benefits to the host.
A. a
B. b
C. c
D. d
![]() Q. 176 Assertion : due to pollution atmospheric concentration of CO₂ is increasing which will be harmful for C₄ plants whereas productive for C₃ plants.
Q. 176 Assertion : due to pollution atmospheric concentration of CO₂ is increasing which will be harmful for C₄ plants whereas productive for C₃ plants.
Reason : C₄ plants have greater efficiency for CO₂ as CO₂ is fixed by PEP oxygenase.
A. a
B. b
C. c
D. d
![]() Q. 177 Assertion : insulin is antagonistic to glucagon.
Q. 177 Assertion : insulin is antagonistic to glucagon.
Reason : It is an anabolic harmone.
A. a
B. b
C. c
D. d
![]() Q. 178 Assertion : auditory ossicles are small bones present in the cavity of inner car.
Q. 178 Assertion : auditory ossicles are small bones present in the cavity of inner car.
Reason : Auditory ossicles maintain the balance of air pressure between two sizes of ear drum.
A. a
B. b
C. c
D. d
![]() Q. 179 Assertion : pharyngeal nephridia play a role in the conservation of water in the earthworm. Reason : they help the earthworm in keeping the skin moist for cutaneous respiration.
Q. 179 Assertion : pharyngeal nephridia play a role in the conservation of water in the earthworm. Reason : they help the earthworm in keeping the skin moist for cutaneous respiration.
A. a
B. b
C. c
D. d
![]() Q. 180 Assertion : pantothenic acid defeciency is probably the most common vitamin deficiency.
Q. 180 Assertion : pantothenic acid defeciency is probably the most common vitamin deficiency.
Reason : Macrocytic anaemia is a characteristic feature of pantothenic acid deficiency.
A. a
B. b
C. c
D. d
![]() Q. 181 Which country has three capitals?
Q. 181 Which country has three capitals?
A. S.Africa
B. switzerland
C. netherland
D. australia
![]() Q. 182 which is the largest desert in the world?
Q. 182 which is the largest desert in the world?
A. atacama
B. thar
C. sahara
D. kalahri
![]() Q. 183 Which is the largest lake in the world?
Q. 183 Which is the largest lake in the world?
A. caspian sea
B. wular
C. lake superior
D. baikal
![]() Q. 184 which country has won ‘Fifa world cup’ maximum times?
Q. 184 which country has won ‘Fifa world cup’ maximum times?
A. germany
B. brazil
C. france
D. italy
![]() Q. 185 ‘world population day’ is on
Q. 185 ‘world population day’ is on
A. 8th marh
B. 21st march
C. 11th july
D. 3rd october
![]() Q. 186 who invented the stethescope?
Q. 186 who invented the stethescope?
A. reni laennec
B. hopkins
C. louis pasteur
D. hausen
![]() Q. 187 which country has largest number of coal reserves?
Q. 187 which country has largest number of coal reserves?
A. USA
B. russia
C. china
D. india
![]() Q. 188 What term is given to the relationship between culture and food?
Q. 188 What term is given to the relationship between culture and food?
A. astronomy
B. agronomy
C. gastronomy
D. geology
![]() Q. 189 one-rupee note bears the signature of the
Q. 189 one-rupee note bears the signature of the
A. governor of reserve bank
B. finance ministry
C. secretary, ministry of finance
D. president of india
![]() Q. 190 Which of the following classical dance forms originated in Andhra Pradesh?
Q. 190 Which of the following classical dance forms originated in Andhra Pradesh?
A. bharatnatyam
B. kathakali
C. kuchipudi
D. odissi
![]() Q. 191 on adding salt to water, the boiling point and freezing point of water will:
Q. 191 on adding salt to water, the boiling point and freezing point of water will:
A. increases
B. increase and decrease respectively
C. decrease
D. decrease and increase respectively
![]() Q. 192 chocolates can be bad for health because of a high contentof
Q. 192 chocolates can be bad for health because of a high contentof
A. cobalt
B. nickel
C. zinc
D. lead
![]() Q. 193 The novel coolie is written by
Q. 193 The novel coolie is written by
A. R.k.Narayan
B. prem chand
C. jainendra kumar
D. mulk raj anand
![]() Q. 194 Beirut is the capital of :
Q. 194 Beirut is the capital of :
A. syria
B. jordan
C. lebanon
D. libya
![]() Q. 195 The first month of saka year is
Q. 195 The first month of saka year is
A. vaisakh
B. chaitra
C. jyeshtha
D. paush
![]() Q. 196 chameli devi award is given to an outstanding woman who is a:
Q. 196 chameli devi award is given to an outstanding woman who is a:
A. vocalist
B. lawyer
C. journalist
D. scientist
![]() Q. 197 “olive branch” is a sign of
Q. 197 “olive branch” is a sign of
A. war
B. peace
C. defeat
D. conquest
![]() Q. 198 800 in roman number is written as
Q. 198 800 in roman number is written as
A. DDCC
B. DDDC
C. DCCC
D. DCCD
![]() Q. 199 santosh trophy is associated with
Q. 199 santosh trophy is associated with
A. hockey
B. cricket
C. badminton
D. football
![]() Q. 200 Which river carries maximum quantity of water in the world?
Q. 200 Which river carries maximum quantity of water in the world?
A. nile
B. amazon
C. thames
D. missisippi
| Question | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Answer | B | C | A | C | B | D | C | B | C | C |
| Question | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Answer | A | C | A | C | C | B | B | A | A | C |
| Question | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Answer | C | A | A | B | C | B | C | A | D | B |
| Question | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 |
| Answer | A | C | B | A | C | B | B | C | C | A |
| Question | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 |
| Answer | A | D | B | D | C | A | A | B | A | A |
| Question | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 |
| Answer | A | C | B | D | A | A | B | C | C | A |
| Question | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 |
| Answer | C | B | A | C | C | A | C | C | C | B |
| Question | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 |
| Answer | C | C | B | B | C | C | C | C | D | B |
| Question | 81 | 82 | 83 | 84 | 85 | 86 | 87 | 88 | 89 | 90 |
| Answer | D | D | C | C | C | A | A | A | C | A |
| Question | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 100 |
| Answer | B | B | A | D | A | D | B | A | B | B |
| Question | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | 109 | 110 |
| Answer | C | B | A | A | C | A | B | C | B | B |
| Question | 111 | 112 | 113 | 114 | 115 | 116 | 117 | 118 | 119 | 120 |
| Answer | A | D | B | B | B | A | A | D | A | C |
| Question | 121 | 122 | 123 | 124 | 125 | 126 | 127 | 128 | 129 | 130 |
| Answer | C | D | D | D | C | A | A | B | A | C |
| Question | 131 | 132 | 133 | 134 | 135 | 136 | 137 | 138 | 139 | 140 |
| Answer | C | D | C | B | B | C | A | C | C | D |
| Question | 141 | 142 | 143 | 144 | 145 | 146 | 147 | 148 | 149 | 150 |
| Answer | C | C | A | D | B | A | C | B | C | B |
| Question | 151 | 152 | 153 | 154 | 155 | 156 | 157 | 158 | 159 | 160 |
| Answer | A | C | C | C | C | B | D | B | C | A |
| Question | 161 | 162 | 163 | 164 | 165 | 166 | 167 | 168 | 169 | 170 |
| Answer | B | B | A | A | D | A | A | D | C | C |
| Question | 171 | 172 | 173 | 174 | 175 | 176 | 177 | 178 | 179 | 180 |
| Answer | C | D | A | B | C | C | A | D | C | D |
| Question | 181 | 182 | 183 | 184 | 185 | 186 | 187 | 188 | 189 | 190 |
| Answer | A | C | A | B | C | A | A | C | C | C |
| Question | 191 | 192 | 193 | 194 | 195 | 196 | 197 | 198 | 199 | 200 |
| Answer | B | D | D | C | B | C | B | C | D | B |