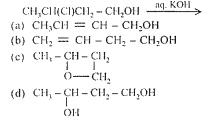![]() AIIMS UG 2009
AIIMS UG 2009
|
Section |
Questions |
Marks |
|
Physics |
60 Questions (1 – 60) |
60 |
|
Chemistry |
60 Questions (61 – 120) |
60 |
|
Biology |
60 Questions (121 – 180) |
60 |
|
General Knowledge |
20 Questions (181 – 200) |
20 |
![]() Question: 1 A convex lens of refractive index 3/2 has a power of 2.5 D in air. It is placed in liquid of refractive index 2 then the new power of the lens is
Question: 1 A convex lens of refractive index 3/2 has a power of 2.5 D in air. It is placed in liquid of refractive index 2 then the new power of the lens is
A. -1.25 D
B. -1.5 D
C. 1.25 D
D. 1.5 D
![]() Q. 2 What is the ratio of Bohr magneton to the nuclear magneton ?
Q. 2 What is the ratio of Bohr magneton to the nuclear magneton ?
A. mᵣ/mₑ
B. mᵣ²/mₑ²
C. 1
D. mₑ/mᵣ
![]() Q. 3 When the inputs of a 2 input logic gate are 0 and 0 and the output is 1.When the inputs are 1 and 0 the output is 0 . The type of logic gate is
Q. 3 When the inputs of a 2 input logic gate are 0 and 0 and the output is 1.When the inputs are 1 and 0 the output is 0 . The type of logic gate is
A. XOR
B. NAND
C. NOR
D. OR
![]() Q. 4 de broglie wavelength λ associated with neutrons is related with absolute temperature T as
Q. 4 de broglie wavelength λ associated with neutrons is related with absolute temperature T as
A. λ∝T
B. λ∝1/T
C. λ∝1/√T
D. λ∝T²
![]() Q. 5 The dimensions of a specific resistance are :
Q. 5 The dimensions of a specific resistance are :
A. ML²T⁻²A⁻¹
B. ML³T⁻³A⁻²
C. ML³T⁻²A⁻¹
D. ML²T⁻²A⁻²
![]() Q. 6 Reciprocal if impedance is
Q. 6 Reciprocal if impedance is
A. Susceptance
B. conductance
C. admittance
D. transconductance
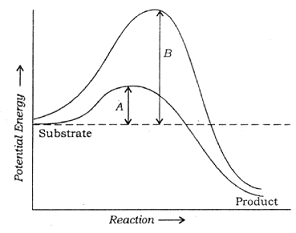
![]() Q. 7 A nucleas of mass number 220 decays by α decay. The energy released in the reaction is 5 mev.The kinetic energy of α particle is :
Q. 7 A nucleas of mass number 220 decays by α decay. The energy released in the reaction is 5 mev.The kinetic energy of α particle is :
A. 1/54 Mev
B. 22/11 Mev
C. 54/11 Mev
D. 55/54 Mev
![]() Q. 8 Four charges are arranged at the corners of a square as shown in the figure .The direction of the electric field at the centre of the square is along
Q. 8 Four charges are arranged at the corners of a square as shown in the figure .The direction of the electric field at the centre of the square is along
A. DC
B. BC
C. AB
D. AD
![]() Q. 9 The wavelengths of kₐ x-rays for lead isotopes Pb²⁰⁸ Pb²⁰⁶ and Pb ²⁰⁴ are λ₁ λ₂ and λ₃
Q. 9 The wavelengths of kₐ x-rays for lead isotopes Pb²⁰⁸ Pb²⁰⁶ and Pb ²⁰⁴ are λ₁ λ₂ and λ₃
respectively . Then
A. λ₂ =√λ₁λ₃
B. λ₂ =λ₁+λ₃
C. λ₂ =λ₁λ₃
D. λ₂ =λ₁/λ₃
![]() Q. 10 A force F acting on an object varies with distance x as shown in the figure . The force is in N and x is m . The work done by the force to move the object from x-=0 to x=6 m is
Q. 10 A force F acting on an object varies with distance x as shown in the figure . The force is in N and x is m . The work done by the force to move the object from x-=0 to x=6 m is
A. 13.5 J
B. 10J
C. 15 J
D. 20 J
![]() Q. 11 Which of the following graph depicts spring constant K versus length L of the spring correctly ?
Q. 11 Which of the following graph depicts spring constant K versus length L of the spring correctly ?
A. A
B. B
C. C
D. D
![]() Q. 12 A body of mass 5 kg moving with a speed 1.5 m/s on a horizontally smooth surface collides with nearly weightless spring of force constant k= 5 N/m. The maximum compression of the spring would be
Q. 12 A body of mass 5 kg moving with a speed 1.5 m/s on a horizontally smooth surface collides with nearly weightless spring of force constant k= 5 N/m. The maximum compression of the spring would be
A. 0.5 m
B. 0.15 m
C. 1.5 m
D. 1.12 m
![]() Q. 13 A body is moved along a straight line by a machine delivering constant power.The distance travelled by a body in the time t is proportional to
Q. 13 A body is moved along a straight line by a machine delivering constant power.The distance travelled by a body in the time t is proportional to
A. t¹⁄2
B. t
C. t³⁄2
D. t²
![]() Q. 14 Light with an energy flux of 18W/cm^2 falls on non reflecting surface at normal incidence .The pressure exerted on the surface is
Q. 14 Light with an energy flux of 18W/cm^2 falls on non reflecting surface at normal incidence .The pressure exerted on the surface is
A. 2 N/M²
B. 2×10⁻⁴N/M²
C. 6N/M²
D. 6×10⁻⁴ N/M²
![]() Q. 15 The dimensional formula of Plancks constant is :
Q. 15 The dimensional formula of Plancks constant is :
A. ML²T⁻¹
B. ML²T⁻²
C. ML⁰T²
D. MLT²
![]() Q. 16 A body is projected horizontally with a velocity of 4√2 m/sec. The velocity of the body after 0.7 sec will be nearly.
Q. 16 A body is projected horizontally with a velocity of 4√2 m/sec. The velocity of the body after 0.7 sec will be nearly.
A. 10 m/sec
B. 9 m /sec
C. 19 m /sec
D. 11 m/sec
![]() Q. 17 Three equal weights of 3 kg each are hanging on a string passing over a frictionless pulley as shown in the figure .The tension in the string between masses II and III will be
Q. 17 Three equal weights of 3 kg each are hanging on a string passing over a frictionless pulley as shown in the figure .The tension in the string between masses II and III will be
A. 5 N
B. 6 N
C. 10 N
D. 20 N
![]() Q. 18 A ball is bouncing down a set of stairs. the coefficient of restitution is e. the height of each step is d and the ball bounces one step at each bounce. after each bounce the ball rebounds to a height h above the next lower step.The height is large enough compared with the width of step so that the impacts are effectively head on .Find the relationship between h and d .
Q. 18 A ball is bouncing down a set of stairs. the coefficient of restitution is e. the height of each step is d and the ball bounces one step at each bounce. after each bounce the ball rebounds to a height h above the next lower step.The height is large enough compared with the width of step so that the impacts are effectively head on .Find the relationship between h and d .
A. A
B. B
C. C
D. D
![]() Q. 19 A conducting sphere of radius R carrying charge Q lies inside the uncharged conducting shell of radius 2R.IF they are joined by metal wire the amount of heat that will be produced is
Q. 19 A conducting sphere of radius R carrying charge Q lies inside the uncharged conducting shell of radius 2R.IF they are joined by metal wire the amount of heat that will be produced is
A. 1/4∐εₒ( Q²/4R)
B. 1/4∐εₒ( Q²/2R)
C. 1/4∐εₒ( Q²/R)
D. 2/4∐εₒ( Q²/3R)
![]() Q. 20 Black holes in orbit around a normal star are detected from the earth due to the frictional heating of infalling gas into the blackhole,which can reach temperature greater than 10⁶K. Assuming that the infalling gas can be modelled as a blackbody radiator , the wavelength of maximum power is
Q. 20 Black holes in orbit around a normal star are detected from the earth due to the frictional heating of infalling gas into the blackhole,which can reach temperature greater than 10⁶K. Assuming that the infalling gas can be modelled as a blackbody radiator , the wavelength of maximum power is
A. in the invisible region
B. in the x ray region
C. in the microwave region
D. in the gamma ray region in electromagnetic spectrum
![]() Q. 21 Neglecting the density of air, the terminal velocity obtained by a raindrop of radius 0.3 mm falling through air of viscosity 1.8 x lO-s N s m2 will be
Q. 21 Neglecting the density of air, the terminal velocity obtained by a raindrop of radius 0.3 mm falling through air of viscosity 1.8 x lO-s N s m2 will be
A. 10.9 m s⁻¹
B. 7.48 m s⁻¹
C. 3.7 m s⁻¹
D. 12.8m s⁻¹
![]() Q. 22 A particle executes simple harmonic motion of period T and amplitude I along a rod AB of length •2L. The rod AB itself executes simple harmonic motion of the same period and amplitude in a direction perpendicular to its length. Initially, both the particle and the rod are in their mean positions. The path traced out by the particle will be
Q. 22 A particle executes simple harmonic motion of period T and amplitude I along a rod AB of length •2L. The rod AB itself executes simple harmonic motion of the same period and amplitude in a direction perpendicular to its length. Initially, both the particle and the rod are in their mean positions. The path traced out by the particle will be
A. a circle of radius I
B. a straight line inclined at∐/4 to the rod
C. an ellipsea
D. figure of eight
![]() Q. 23 What is the energy stored in the capacitor between terminals a and b of the network shown . in the figure? (Capacitance of each capacitance c = 1μF)
Q. 23 What is the energy stored in the capacitor between terminals a and b of the network shown . in the figure? (Capacitance of each capacitance c = 1μF)
A. 12.5 μJ
B. 0 μJ
C. 25 μJ
D. 50 μJ
![]() Q. 24 When a current is passed in a conductor, 3°C rise in temperature is observed. If the
Q. 24 When a current is passed in a conductor, 3°C rise in temperature is observed. If the
strength of current is made thrice, then rise in temperature will approximately be
A. 36 °C
B. 27°C
C. 18°C
D. 9°C
![]() Q. 25 In a metal with positive Thomson coefficient, current is passed from the lower temperature to higher temperature side. Then heat will be
Q. 25 In a metal with positive Thomson coefficient, current is passed from the lower temperature to higher temperature side. Then heat will be
A. absorbed
B. constant
C. evolved
D. either b or c
![]() Q. 26 A moving coil galvanometer has a resistance of 900 Q. In order to send only 10% of the main current through this galvanometer, the resistance of the required shunt is
Q. 26 A moving coil galvanometer has a resistance of 900 Q. In order to send only 10% of the main current through this galvanometer, the resistance of the required shunt is
A. 0.9.Q
B. 100 Q
C. 405 Q
D. 90 Q
![]() Q. 27 A current 11 carrying wire AB is placed near another long wire CD carrying current l₂ If wire AB is free to move, it will have
Q. 27 A current 11 carrying wire AB is placed near another long wire CD carrying current l₂ If wire AB is free to move, it will have
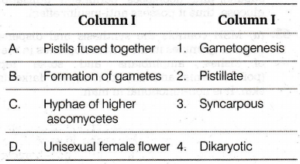
A. rotational motion only
B. translational motion only
C. rotational as well as translational motion
D. neither rotational nor translational motion
![]() Q. 28 A coil of wire of a certain radius has 100 turns and a self inductance of 15 mH: The self inductance of a second similar coil of 500 turns will be
Q. 28 A coil of wire of a certain radius has 100 turns and a self inductance of 15 mH: The self inductance of a second similar coil of 500 turns will be
A. 75 mH
B. 375 mH
C. 15 mH
D. none of these
![]() Q. 29 In a series LCR circuit the voltage across the resistance, capacitance and inductance is 10 V each. If the capacitance is short circuited the voltage across the inductance will be
Q. 29 In a series LCR circuit the voltage across the resistance, capacitance and inductance is 10 V each. If the capacitance is short circuited the voltage across the inductance will be
A. 10 v
B. 10√2 v
C. 10/√2 v
D. 20 v
![]() Q. 30 If Vₐ Vₓ and Vᵣn are the speeds of gamma rays, X-rays and microwaves respectively in vacuum, then.
Q. 30 If Vₐ Vₓ and Vᵣn are the speeds of gamma rays, X-rays and microwaves respectively in vacuum, then.
A. Vₐ >Vₓ > Vᵣn
B. Vₐ < Vₓ < Vᵣn
C. Vₐ >Vₓ > Vᵣn
D. Vₐ = Vₓ = Vᵣn
![]() Q. 31 Which out of following, cannot produce two coherent sources?
Q. 31 Which out of following, cannot produce two coherent sources?
A. Lloyd’s mirror
B. Fresnel biprism
C. Young’s double slit
D. Prism
![]() Q. 32 In Young’s double slit experiment, the two slits act as coherent sources of equal amplitude a and of wavelength λ. In another experiment with the same set up, the two slits are sources of equal amplitude a and wavelength A, but are incoherent. The ratio of intensities of light at the mid point of the screen in the first case to that in the second case is
Q. 32 In Young’s double slit experiment, the two slits act as coherent sources of equal amplitude a and of wavelength λ. In another experiment with the same set up, the two slits are sources of equal amplitude a and wavelength A, but are incoherent. The ratio of intensities of light at the mid point of the screen in the first case to that in the second case is
A. 2:1
B. 1:2
C. 3:4
D. 4:3
![]() Q. 33 If the kinetic energy of a particle is increased by 16 times, the percentage change in the de Broglie wavelength of the particle is
Q. 33 If the kinetic energy of a particle is increased by 16 times, the percentage change in the de Broglie wavelength of the particle is
A. 25%
B. 75%
C. 60%
D. 50%
![]() Q. 34 If the half lives of a radioactive element for α and β decay are 4 years and 12 years
Q. 34 If the half lives of a radioactive element for α and β decay are 4 years and 12 years
respectively, the ratio of its inital activity and that after 12 years will be
A. 6.25%
B. 12.5%
C. 25%
D. 50%
![]() Q. 35 In the given circuit, the potential difference between A and B is
Q. 35 In the given circuit, the potential difference between A and B is
A. 0
B. 5 volt
C. 10 volt
D. 15 volt
![]() Q. 36 A ball is suspended by a thread of length L at the point O on a wall which is inclined to the vertical by α. The thread with the ball is displaced by a small angle β away from the vertical and also away from the wall. If the ball is released, the period of oscillation of the pendulum when β> α.will be
Q. 36 A ball is suspended by a thread of length L at the point O on a wall which is inclined to the vertical by α. The thread with the ball is displaced by a small angle β away from the vertical and also away from the wall. If the ball is released, the period of oscillation of the pendulum when β> α.will be
A. A
B. B
C. C
D. D
![]() Q. 37 A radioactive nucleus is being produced at a constant rate α per second. Its decay constant is λ .If N₀ re the number of nuclei at time t = 0, then the maximum number of nuclei possible are
Q. 37 A radioactive nucleus is being produced at a constant rate α per second. Its decay constant is λ .If N₀ re the number of nuclei at time t = 0, then the maximum number of nuclei possible are
A. A
B. B
C. C
D. D
![]() Q. 38 Awire of length 1 and mass m is bent in the form of a semicircle. The gravitational field intensity at the centre of semicircle is
Q. 38 Awire of length 1 and mass m is bent in the form of a semicircle. The gravitational field intensity at the centre of semicircle is
A. A
B. B
C. C
D. D
![]() Q. 39 In a concave mirror, an object is placed at a distance d1 from the focus and the image is formed at a distance dz from the focus. Then the focal length of the mirror is
Q. 39 In a concave mirror, an object is placed at a distance d1 from the focus and the image is formed at a distance dz from the focus. Then the focal length of the mirror is
A. √d₁d₂
B. d₁d₂
C. (d₁+d₂)/2
D. √d₁/d₂
![]() Q. 40 A short linear object, of length I, lies along the axis of a concave mirror, of focal length f, at a distance d from the pole of the mirror. The size of the image is then (nearly)
Q. 40 A short linear object, of length I, lies along the axis of a concave mirror, of focal length f, at a distance d from the pole of the mirror. The size of the image is then (nearly)
A. lf/(d-f)
B. (d-f)/lf
C. l(f²)/(d-f)²
D. (d-f)²/l(f²)
![]() Questions: 41 – 60
Questions: 41 – 60
In the following questions (41·60), a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as :
![]() Q. 41 Assertion: Liquid molecules have greater potential energy at the melting point.
Q. 41 Assertion: Liquid molecules have greater potential energy at the melting point.
Reason : Intermolecular spacing between molecules increases at melting point.
A. A
B. B
C. C
D. D
![]() Q. 42 Assertion: The bob of a simple pendulum is a ball full of water, if a fine hole is made in the bottom of the ball, the time period first increases and then decreases.
Q. 42 Assertion: The bob of a simple pendulum is a ball full of water, if a fine hole is made in the bottom of the ball, the time period first increases and then decreases.
Reason : As water flows out of the bob the weight of bob decreases
A. A
B. B
C. C
D. D
![]() Q. 43 Assertion: Electric potential of earth is taken zero.
Q. 43 Assertion: Electric potential of earth is taken zero.
Reason : No electric field exists on earth surface.
A. A
B. B
C. C
D. D
![]() Q. 44 Assertion: A charge, whether stationary or in motion produces a magnetic field around it.
Q. 44 Assertion: A charge, whether stationary or in motion produces a magnetic field around it.
Reason : Moving charges produce only electric. field in the surrounding space.
A. A
B. B
C. C
D. D
![]() Q. 45 Assertion: Magnetic susceptibillity is a pure number.
Q. 45 Assertion: Magnetic susceptibillity is a pure number.
Reason : The value of magnetic susceptibility • for vacuum is one.
A. A
B. B
C. C
D. D
![]() Q. 46 Assertion: Transformers are used only in alternating current source not in direct current.
Q. 46 Assertion: Transformers are used only in alternating current source not in direct current.
Reason : Only a.c, can be stepped up or down by means of transformers.
A. A
B. B
C. C
D. D
![]() Q. 47 Assertion: A total reflecting prism is used to erect the inverted image without deviation
Q. 47 Assertion: A total reflecting prism is used to erect the inverted image without deviation
Reason : Rays of light incident parallel to base of prism emerge out as parallel rays.
A. A
B. B
C. C
D. D
![]() Q. 48 Assertion: The edges of the images of white object formed by a concave mirror on the screen appear white.
Q. 48 Assertion: The edges of the images of white object formed by a concave mirror on the screen appear white.
Reason : Concave mirror does not suffer chromatic aberration.
A. A
B. B
C. C
D. D
![]() Q. 49 Assertion: A photon has no rest mass, yet it carries definite momentum.
Q. 49 Assertion: A photon has no rest mass, yet it carries definite momentum.
Reason : Momemtum of photon is due to its energy and hence its equivalent mass.
A. A
B. B
C. C
D. D
![]() Q. 50 Assertion: A photocell is called an electric eye.
Q. 50 Assertion: A photocell is called an electric eye.
Reason : When light is incident on some semiconductor its electrical resistance is reduced.
A. A
B. B
C. C
D. D
![]() Q. 51 Assertion: Nuclei having number about 60 are most stable.
Q. 51 Assertion: Nuclei having number about 60 are most stable.
Reason : When two or more light nuclei are combined into a heavier nucleus, then the
binding energy per nucleon will increase.
A. A
B. B
C. C
D. D
![]() Q. 52 Assertion: The edges of the images of white object formed by a concave mirror on the screen appear white.
Q. 52 Assertion: The edges of the images of white object formed by a concave mirror on the screen appear white.
Reason : Concave mirror does not suffer chromatic aberration.
A. A
B. B
C. C
D. D
![]() Q. 53 Assertion : Most amplifiers use common emitter circuit configuration.
Q. 53 Assertion : Most amplifiers use common emitter circuit configuration.
Reason : Its input resistance is comparatively higher.
A. A
B. B
C. C
D. D
![]() Q. 54 Assertion: For an isothermal process in an ideal gas, the heat obsorbed by the gas is entirely used in the work done by the gas.
Q. 54 Assertion: For an isothermal process in an ideal gas, the heat obsorbed by the gas is entirely used in the work done by the gas.
Reason : During a process taking place in a system, the temperature remains constant
then the process is isothermal.
A. A
B. B
C. C
D. D
![]() Q. 55 Assertion: When hot water is poured in a beaker of thick glass, the beaker cracks.
Q. 55 Assertion: When hot water is poured in a beaker of thick glass, the beaker cracks.
Reason : Outer surface of the beaker expands suddenly.
A. A
B. B
C. C
D. D
![]() Q. 56 Assertion; Generally the path of a projectile from the earth is parabolic but it is elliptical for projectiles going to a very great height.
Q. 56 Assertion; Generally the path of a projectile from the earth is parabolic but it is elliptical for projectiles going to a very great height.
Reason : Up to ordinary height the projectile moves under a uniform gravitational force,
but for great heights, projectile moves under a variable force.
A. A
B. B
C. C
D. D
![]() Q. 57 Assertion: Angular speed of a planet around the sun increases, when it is closer to the sun.
Q. 57 Assertion: Angular speed of a planet around the sun increases, when it is closer to the sun.
Reason : Total angular momentum of the system remains constant.
A. A
B. B
C. C
D. D
![]() Q. 58 Assertion: The size and shape of the rigid body remains unaffected under the effect of external forces.
Q. 58 Assertion: The size and shape of the rigid body remains unaffected under the effect of external forces.
Reason : The distance bet”veen two particles remains constant in a rigid body.
A. A
B. B
C. C
D. D
![]() Q. 59 Assertion: Impulsive force is large and acts for a short time.
Q. 59 Assertion: Impulsive force is large and acts for a short time.
Reason : Finite change in momentum should be produced by the force.
A. A
B. B
C. C
D. D
![]() Q. 60 Assertion: The dimensional formula for product of resistance and conductance is same as for dielectric constant.
Q. 60 Assertion: The dimensional formula for product of resistance and conductance is same as for dielectric constant.
Reason : Both have dimensions of time constant.
A. A
B. B
C. C
D. D
![]() Q. 61 KF combines with HF to form KHF2 . This compound contains the species
Q. 61 KF combines with HF to form KHF2 . This compound contains the species
A. K⁺ F⁻ and H⁺
B. K⁺ F⁻ and HF
C. K⁺ and (HF₂)⁻
D. (KHF)⁺ and F₂
![]() Q. 62 For a dilute solution, Raoult’s law states that
Q. 62 For a dilute solution, Raoult’s law states that
A. the relative lowering of vapour pressure is proportional to the amount of solute in solution
B. The relative lowering of vapour pressure is equal to the mole fraction of solute
C. The lowering of vapour pressure is equal to the mole fraction of the solute
D. the vapour pressure of the solution is equal to the mole fraction of the solvent
![]() Q. 63 To a 25 ml H₂O₂ olution, excess of acidified solution of KI was added. The iodine liberated required 20 ml of 0.3 N Na₂S₂O₃ solution.The volume strength of H₂O₂ solution is
Q. 63 To a 25 ml H₂O₂ olution, excess of acidified solution of KI was added. The iodine liberated required 20 ml of 0.3 N Na₂S₂O₃ solution.The volume strength of H₂O₂ solution is
A. 1.344 g/L
B. 3.244 g/L
C. 5.4 g/L
D. 4.08 g/L
![]() Q. 64 Which of the following shows bond in silicone?
Q. 64 Which of the following shows bond in silicone?
A. Si-C-Si-C-Si
B. si-si-si-si
C. Si-O-Si-O-Si
D. Si-C-Si-0-Si
![]() Q. 65 pH of a 0.01 M solution (Kₐ=6.6 ×10⁻⁴)
Q. 65 pH of a 0.01 M solution (Kₐ=6.6 ×10⁻⁴)
A. 7.6
B. 8
C. 2.6
D. 5
![]() Q. 66 In a homogenous reaction A −> B + C + D the initial pressure was Po and after time t it was • P. Expression for rate constant k in terms of Po pand t will be
Q. 66 In a homogenous reaction A −> B + C + D the initial pressure was Po and after time t it was • P. Expression for rate constant k in terms of Po pand t will be
A. A
B. B
C. C
D. D
![]() Q. 67 Which curve corresponds to the temperature dependance of the rate R of a simple one step reaction?
Q. 67 Which curve corresponds to the temperature dependance of the rate R of a simple one step reaction?
A. A
B. B
C. C
D. D
![]() Q. 68 A vessel of one litre capacity containing 1 mole of S03 is heated till a state of equilibrium is attained. At equilibrium, 0.6 moles of SO2 had formed. The value of equilibrium constant is
Q. 68 A vessel of one litre capacity containing 1 mole of S03 is heated till a state of equilibrium is attained. At equilibrium, 0.6 moles of SO2 had formed. The value of equilibrium constant is
A. 0.18
B. 0.36
C. 0.45
D. 0.68
![]() Q. 69 A 0.1 molal solution of an acid is 4.5% ionized. Calculate freezing point. (molecular weight of the acid is 300). Kf = 1.86 K mol⁻¹ kg.
Q. 69 A 0.1 molal solution of an acid is 4.5% ionized. Calculate freezing point. (molecular weight of the acid is 300). Kf = 1.86 K mol⁻¹ kg.
A. -0.199°C
B. 2.00°C
C. 0°C
D. -0.269°C
![]() Q. 70 Which of the following is an example of chain silicates?
Q. 70 Which of the following is an example of chain silicates?
A. Kaolinite
B. Zircon
C. Benitonite
D. Diopside
![]() Q. 71 Which of the element shows +4 oxidation state?
Q. 71 Which of the element shows +4 oxidation state?
A. Sn
B. Ra
C. Fr
D. Sc
![]() Q. 72 Tincture of iodine is
Q. 72 Tincture of iodine is
A. aqueous solution of I₂
B. solution of I₂ in aqueous K
C. alcoholic solution of I₂
D. aqueous solution of KI
![]() Q. 73 The specific conductance of a N/10 KCI at 25°C is 0.0112 ohm⁻¹ cm⁻¹The resistance of cell containing solution at the same temperature was found to be 55 ohm. The cell constant will be
Q. 73 The specific conductance of a N/10 KCI at 25°C is 0.0112 ohm⁻¹ cm⁻¹The resistance of cell containing solution at the same temperature was found to be 55 ohm. The cell constant will be
A. 6.16 cm⁻¹
B. 0.616 cm⁻¹
C. 0.0616 cm⁻¹
D. 616 cm⁻¹
![]() Q. 74 Decreasing order of stability of ions is
Q. 74 Decreasing order of stability of ions is
A. A
B. B
C. C
D. D
![]() Q. 75 What is A?
Q. 75 What is A?
A. a
B. b
C. c
D. d
![]() Q. 76 What is B?
Q. 76 What is B?
A. a
B. b
C. c
D. d
![]() Q. 77 What is D?
Q. 77 What is D?
A. a
B. b
C. c
D. d
![]() Q. 78 Mercurous chloride exists in the form of
Q. 78 Mercurous chloride exists in the form of
A. Hg⁺
B. Hg²⁺
C. Hg₂²⁺
D. Hg₃²⁺
![]() Q. 79 Formula of microcosmic salt is
Q. 79 Formula of microcosmic salt is
A. Na₂HPO₄
B. Na(NH₄)HPO₄
C. K₂HPO₄
D. Na₂PO₄ . K₂PO₄
![]() Q. 80 What is the molarity of H₂SO₄ solution that has a density of 1.84 g/cc at 35°C and contains 98% by weight?
Q. 80 What is the molarity of H₂SO₄ solution that has a density of 1.84 g/cc at 35°C and contains 98% by weight?
A. 4.18 M
B. 8.14 M
C. 18.4 M
D. 18 M
![]() Q. 81 A mixture of two miscible liquids A and B is distilled under equilibrium conditions at 1 atm pressure. The mole fraction of A in solution and vapour phase are 0.30 and 0.60 respectively. Assuming ideal behaviour of the solution and the vapour, calculate the ratio of the vapour pressure of pure A to that of pure B.
Q. 81 A mixture of two miscible liquids A and B is distilled under equilibrium conditions at 1 atm pressure. The mole fraction of A in solution and vapour phase are 0.30 and 0.60 respectively. Assuming ideal behaviour of the solution and the vapour, calculate the ratio of the vapour pressure of pure A to that of pure B.
A. 4.0
B. 3.5
C. 2.5
D. 1.85
![]() Q. 82 The variation of volume V, with temperature T, keeping pressure constant is called the coefficient of thermal expansion (a) of a gas. i.e. α= 1/V(∂Y/∂T)ₓ. For an ideal gas α is equal to :
Q. 82 The variation of volume V, with temperature T, keeping pressure constant is called the coefficient of thermal expansion (a) of a gas. i.e. α= 1/V(∂Y/∂T)ₓ. For an ideal gas α is equal to :
A. T
B. 1/T
C. P
D. 1/P
![]() Q. 83 The molecules having the same hybridization, shape and number of lone pairs of electrons are
Q. 83 The molecules having the same hybridization, shape and number of lone pairs of electrons are
A. SeF₄, XeO₂F₂
B. SF₄, XeF₂
C. XeOF₄, TeF₄
D. XeCl₄, XeF₄
![]() Q. 84 The correct order of stability of the superoxides is
Q. 84 The correct order of stability of the superoxides is
A. KO₂ > RbO₂ > CsO₂
B. KO₂ > CsO₂ > RbO₂
C. CsO₂ > RbO₂ > KO₂
D. RbO₂ > CsO₂ > KO₂
![]() Q. 85 Schottky defect in crystals is observed when
Q. 85 Schottky defect in crystals is observed when
A. unequal number cations and anions are missing from the lattice
B. equal number of cations and anions are missing from the lattice
C. an ion leaves its normal site and occupies an interstitial site
D. density of the crystal is increased
![]() Q. 86 Consider the reaction
Q. 86 Consider the reaction
The correct explanation is
A. The product is formed due to nucleophilic substitution
B. The product is formed according to Saytzeff’s rule
C. The product is formed according to Saytzeff’s rule
D. (CH₃)₃CO⁻ is a better leaving group
![]() Q. 87 2.5 g of the carbonate of a metal was treated with 100ml of IN H2S04, After the completion of the reaction, the solution was boiled off to expel CO2 and was then titrated against IN NaOH solution. The volume of alkali that would be consumed, if the equivalent weight of the metal is 20
Q. 87 2.5 g of the carbonate of a metal was treated with 100ml of IN H2S04, After the completion of the reaction, the solution was boiled off to expel CO2 and was then titrated against IN NaOH solution. The volume of alkali that would be consumed, if the equivalent weight of the metal is 20
A. 50
B. 25
C. 75
D. 100
![]() Q. 88 In solvents like DMSO, acetonitrile, F⁻ ion of dissolved NaF is more reactive than in methyl alcohol. Explain
Q. 88 In solvents like DMSO, acetonitrile, F⁻ ion of dissolved NaF is more reactive than in methyl alcohol. Explain
A. CH₃OH is more polar than DMSO and CH₃CN
B. CH₃OH is less polar than DMSO and CH₃CN
C. unsolved F⁻ ion is DMSO or CH₃CN acts more efficiently as nucleophile
D. -OH group is a better leaving group than F -ion
![]() Q. 89 Which of the following fluorides has the lowest melting point?
Q. 89 Which of the following fluorides has the lowest melting point?
A. BaF₂
B. SrF₂
C. CaF₂
D. BeF₂
![]() Q. 90 Which of the following has the highest tendency to give the reaction
Q. 90 Which of the following has the highest tendency to give the reaction
A. Na
B. Li
C. K
D. Rb
![]() Q. 91 How many geometrical isomers are possible in the following two alkanes ?
Q. 91 How many geometrical isomers are possible in the following two alkanes ?
(i) CH3 – CH =CH – CH = CH – CH3
(ii) CH3-CH= CH -CH = CH -Cl
A. 4 and 4
B. 4 and 3
C. 3 and 3
D. 3 and 4
![]() Q. 92 The equilibrium constant for mutarotation α-D Glucose ⇔ β-D Glucose 1.8. What
Q. 92 The equilibrium constant for mutarotation α-D Glucose ⇔ β-D Glucose 1.8. What
percentage of α form remains at equilibrium?
A. 35.7
B. 64.3
C. 55.6
D. 44.4
![]() Q. 93 2-phenylethylbromide when heated with NaOEt, elimination takes place. No deuterium exchange takes place when the reaction is carried out in C₂H₅OD solvent. The mechanism will be
Q. 93 2-phenylethylbromide when heated with NaOEt, elimination takes place. No deuterium exchange takes place when the reaction is carried out in C₂H₅OD solvent. The mechanism will be
A. E1 elimination
B. E2 elimination
C. E1cB elimination
D. E2 or E1cB
![]() Q. 94 The M – O – M bond angles in M₂O (where M is halogen) is in the order
Q. 94 The M – O – M bond angles in M₂O (where M is halogen) is in the order
A. Br₂O > Cl₂O > F₂O
B. F₂O > Br₂O > Cl₂O
C. F₂O > Cl₂O > Br₂O
D. Cl₂O > F₂O > Br₂O
![]() Q. 95 Hydroflouric acid is a weak acid. At 25°C, the molar conductivity of 0.002 M HF is 176.2 Ω⁻¹ cm² mol⁻¹. If its Λ°m = 405.2 Ω⁻¹ cm² mol⁻¹. Equilibrium constant at the given concentration is
Q. 95 Hydroflouric acid is a weak acid. At 25°C, the molar conductivity of 0.002 M HF is 176.2 Ω⁻¹ cm² mol⁻¹. If its Λ°m = 405.2 Ω⁻¹ cm² mol⁻¹. Equilibrium constant at the given concentration is
A. 6.7 x 10⁻⁴ M
B. 3.2 x 10⁻⁴ M
C. 6.7 x 10⁻⁵ M
D. 3.2 x 10⁻⁵ M
![]() Q. 96 In Oppenauer’s oxidation,
Q. 96 In Oppenauer’s oxidation,
A. secondary alcohol is oxidised to carboxylic acid in acetone solvent using aluminium tertiary butaoxide
B. secondary alcohol is oxidised to carboxylic acid without affecting the C = C or C ≡ C bond buy aluminium tertiary butaoxide in acetone solvent
C. secondary alcohol is oxidised to ketone without affecting C = C or C ≡ C bond by aluminium tertiary butaoxide
D. secondary alcohol is oxidised to ketone by chromic acid – pyridine complex.
![]() Q. 97 Incorrect statement about Ge is
Q. 97 Incorrect statement about Ge is
A. GeO₂ is weakly acidic
B. Ge(OH)₂ s amphoteric
C. GeCl₂ is more stable than GeCl₄
D. Ge – Ge bond energy is lesser than that of Si – Si
![]() Q. 98 In an isobaric process, when temperature changes from T₁ to T₂ ΔS is equal to
Q. 98 In an isobaric process, when temperature changes from T₁ to T₂ ΔS is equal to
A. 2.303 Cp log (T₂/T₁)
B. 2.303 Cp ln (T₂/T₁)
C. Cp ln (T₁/T₂)
D. Cv ln (T₂/T₁)
![]() Q. 99 In the given reaction A and B are ?
Q. 99 In the given reaction A and B are ?
A. a
B. b
C. c
D. d
![]() Q. 100 In the following sequence of the reactions, identify the final product.
Q. 100 In the following sequence of the reactions, identify the final product.
A. a
B. b
C. c
D. d
![]() Questions: 101 – 120
Questions: 101 – 120
In the following questions (101-120), a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as :
(a) If both assertion and reason are true and reason is the correct explanation of assertion
(b) If both assertion and reason are true but reason is not the correct explanation of assertion
(c) If assertion is true but reason is false
(d) If both assertion and reason are false.
![]() Q. 101 Assertion: Electromeric effect is brought into play only at the requirement of the reagent.
Q. 101 Assertion: Electromeric effect is brought into play only at the requirement of the reagent.
Reason : It is a temporary effect in which bond pair is shifted to one of the constituent atoms.
A. A
B. B
C. C
D. D
![]() Q. 102 Assertion: In fused state, calcium chloride cannot be used to dry alcohol or NH3.
Q. 102 Assertion: In fused state, calcium chloride cannot be used to dry alcohol or NH3.
Reason : CaCI2 is not a good dessicant.
A. A
B. B
C. C
D. D
![]() Q. 103 Assertion: Heat of neutralisation of nitric acid with NaOH is same to that of HCl and NaOH.
Q. 103 Assertion: Heat of neutralisation of nitric acid with NaOH is same to that of HCl and NaOH.
Reason : In both cases strong add and strong bases are neutralised.
H⁺+OH⁻ −> H₂O
A. A
B. B
C. C
D. D
![]() Q. 104 Assertion: Cis-2-butene gives meso-2 ,3-butanediol with dilute alkaline KMn04 solution.
Q. 104 Assertion: Cis-2-butene gives meso-2 ,3-butanediol with dilute alkaline KMn04 solution.
Reason : Dilute alkaline KMn04 solution gives trans addition with alkenes.
A. A
B. B
C. C
D. D
![]() Q. 105 Assertion: Ethers can be dried by using sodium wire.
Q. 105 Assertion: Ethers can be dried by using sodium wire.
Reason : Ethers do not react with sodium.
A. A
B. B
C. C
D. D
![]() Q. 106 Assertion: In rate law, unlike in the expression for equilibrium constants, the exponents for concentrations do not necessarily match the stoichiometric coefficients.
Q. 106 Assertion: In rate law, unlike in the expression for equilibrium constants, the exponents for concentrations do not necessarily match the stoichiometric coefficients.
Reason : It is the mechanism and not the balanced chemical equation for the overall
change that governs the reaction rate
A. A
B. B
C. C
D. D
![]() Q. 107 Assertion: The presence of Ag” enhances the solubility of alkenes in water.
Q. 107 Assertion: The presence of Ag” enhances the solubility of alkenes in water.
Reason : Alkenes are weakly polar in nature.
A. A
B. B
C. C
D. D
![]() Q. 108 Assertion: A reaction which is spontaneous and accompanied by decrease of randomness must be exothermic.
Q. 108 Assertion: A reaction which is spontaneous and accompanied by decrease of randomness must be exothermic.
Reason : All exothermic reactions are accompanied by decrease of randomness.
A. A
B. B
C. C
D. D
![]() Q. 109 Assertion: Compressibility factor for hydrogen varies with pressure with positive slope at all pressure.
Q. 109 Assertion: Compressibility factor for hydrogen varies with pressure with positive slope at all pressure.
Reason : Even at low pressure, repulsive forces dominate for hydrogen gas
A. A
B. B
C. C
D. D
![]() Q. 110 Assertion: p-N,N-ditncthylaminobenzaldehyde. undergoes benzoin condensation.
Q. 110 Assertion: p-N,N-ditncthylaminobenzaldehyde. undergoes benzoin condensation.
Reason : The aldehydic (-CHO) group is meta directing.
A. A
B. B
C. C
D. D
![]() Q. 111 Assertion: The S-S-S bond angle in 58 molecule is 105!!.
Q. 111 Assertion: The S-S-S bond angle in 58 molecule is 105!!.
Reason : S8 has a V-shape.
A. A
B. B
C. C
D. D
![]() Q. 112 Assertion: Sodium formate has both the C – 0 bonds have same value 1.27 A.
Q. 112 Assertion: Sodium formate has both the C – 0 bonds have same value 1.27 A.
Reason : Equal bond length is due to the phenomenon of resonance
A. A
B. B
C. C
D. D
![]() Q. 113 Assertion: C2H5Brreacts with alcoholic solution of AgN02 to form nitroethanc as the major product.
Q. 113 Assertion: C2H5Brreacts with alcoholic solution of AgN02 to form nitroethanc as the major product.
Reason : NO;’ is an ambidient ion.
A. A
B. B
C. C
D. D
![]() Q. 114 Assertion: Ice ~ water, if pressure is applied water will evaporate.
Q. 114 Assertion: Ice ~ water, if pressure is applied water will evaporate.
Reason : Increases of pressure pushes the equilibrium towards the side in which number of gilseous molecule increases
A. A
B. B
C. C
D. D
![]() Q. 115 Assertion: Ebonite is highly vulcanised rubber.
Q. 115 Assertion: Ebonite is highly vulcanised rubber.
Reason : Perlon is used in the manufacture of fibres
A. A
B. B
C. C
D. D
![]() Q. 116 Assertion: Al forms (AlF₆)⁻³ but doesnot form (BF₆)⁻³
Q. 116 Assertion: Al forms (AlF₆)⁻³ but doesnot form (BF₆)⁻³
Reason : B does not react with fluorine
A. A
B. B
C. C
D. D
![]() Q. 117 Assertion: Esters which contain a-hydrogensundergo Claisen condensation.
Q. 117 Assertion: Esters which contain a-hydrogensundergo Claisen condensation.
Reason : LiAlH4 reduction of esters gives acid
A. A
B. B
C. C
D. D
![]() Q. 118 Assertion: In an acid-base titration involving strong base and a weak acid, methyl orange can be used as an indicator.
Q. 118 Assertion: In an acid-base titration involving strong base and a weak acid, methyl orange can be used as an indicator.
Reason : Methyl orange changes its colour in pH range of 7 to 9
A. A
B. B
C. C
D. D
![]() Q. 119 Assertion: Millon’s test is a test for identification of proteins.
Q. 119 Assertion: Millon’s test is a test for identification of proteins.
Reason : Millon’s reagent is a solution of mercurous nitrate and mercuric nitrate in nitrite acid containing little nitrous acid
A. A
B. B
C. C
D. D
![]() Q. 120 Assertion: Cu(OH)₂ is soluble in NH40H but not in NaOH
Q. 120 Assertion: Cu(OH)₂ is soluble in NH40H but not in NaOH
Reason : Cu(OH)₂ forms a soluble complexwith NH
A. A
B. B
C. C
D. D
![]() Q. 121 What is diapedesis?
Q. 121 What is diapedesis?
A. a kind of amoeboid movement
B. the process of filtration of urea in kidney
C. a type of locomotion found in Hydra
D. migration of WBCs into the tissue spaces from blood capillaries
![]() Q. 122 Which one of the following depresses brain activity and produces feelings of calmness, relaxation and drowsiness?
Q. 122 Which one of the following depresses brain activity and produces feelings of calmness, relaxation and drowsiness?
A. morphine
B. valium
C. amphetamines
D. hashish
![]() Q. 123 Which of the following nerves arises from organ of Corti?
Q. 123 Which of the following nerves arises from organ of Corti?
A. olfatory nerve
B. cochlear nerve
C. abducens nerve
D. none of these
![]() Q. 124 Delicate hair-like feathers which remain sparsely distributed over the body arc
Q. 124 Delicate hair-like feathers which remain sparsely distributed over the body arc
A. coverts
B. filoplumes
C. plumules
D. remiges
![]() Q. 125 Homo erccius evolved during erccius evolved during
Q. 125 Homo erccius evolved during erccius evolved during
A. pleistocene
B. miocene
C. pliocene
D. holocene
![]() Q. 126 Which of the following postulates is related with Neo-Darwinism?
Q. 126 Which of the following postulates is related with Neo-Darwinism?
A. mutations are believed to help form new species
B. it incorporates isolation as an essential component of evolution
C. it can explain the occurrence of unchanged forms over millions of years
D. all of the above
![]() Q. 127 Intermediate host is absent in the infection of
Q. 127 Intermediate host is absent in the infection of
A. plasmodium
B. Trypanosoma
C. entamoeba
D. filarial worm
![]() Q. 128 Which one feature is common to leech, cockroach and scorpion?
Q. 128 Which one feature is common to leech, cockroach and scorpion?
A. nephridia
B. ventral nerve cord
C. cephalization
D. antennae
![]() Q. 129 The type of epithelial cells which line the inner surface of Fallopian tubes, bronchioles and small bronchi are known a
Q. 129 The type of epithelial cells which line the inner surface of Fallopian tubes, bronchioles and small bronchi are known a
A. squamous epithelium
B. columnar epithelium
C. ciliated epithelium
D. cuboidal epithelium
![]() Q. 130 A person who shows unpredictable moods, outbursts of emotions, quarrelsome behaviour and conflicts with others is suffering from
Q. 130 A person who shows unpredictable moods, outbursts of emotions, quarrelsome behaviour and conflicts with others is suffering from
A. borderline personality disorder (BPD
B. mood disorder
C. addictive disorder
D. schizophrenia
![]() Q. 131 In humans at the end of first meiotic division, the male germ cells differentiate into the
Q. 131 In humans at the end of first meiotic division, the male germ cells differentiate into the
A. primary sperrnatocytes
B. secondary spermatocytes
C. spermatids
D. spermatogonia
![]() Q. 132 Contraceptive oral pills help in birth control by
Q. 132 Contraceptive oral pills help in birth control by
A. killing the sperms in uterus
B. preventing implantation
C. reventing ovulation
D. both b and c
![]() Q. 133 Which one of the following is a sesamoid bone?
Q. 133 Which one of the following is a sesamoid bone?
A. pelvis
B. patella
C. pterygoid
D. pectoral girdle.
![]() Q. 134 Respiration is controlled by
Q. 134 Respiration is controlled by
A. medulla oblongata
B. cerebellum
C. hypothalamus
D. cerebrum
![]() Q. 135 Duodenum has characteristic Brunner’s gland which secretes two hormones called
Q. 135 Duodenum has characteristic Brunner’s gland which secretes two hormones called
A. prolactin, parathormone
B. ecretin, cholecystokinin
C. nterocrinin, duocrinin
D. gastrin, euterogastrone.
![]() Q. 136 Which 0 f the follow ing species has the chromosome complement similar to that of Triticum aestiuum?
Q. 136 Which 0 f the follow ing species has the chromosome complement similar to that of Triticum aestiuum?
A. Zen mays
B. Scenic cercale
C. Gosefpium
D. egilope
![]() Q. 137 Eugenics is the branch concerned with
Q. 137 Eugenics is the branch concerned with
A. improving the quality of human race by symptomatic treatment of genetic diseases
B. improving the quality of human populations by the application of genetic principles
C. improving the quality of human race by providing best suitable environment
D. none of these
![]() Q. 138 What is incorrect about the following figure representing DNA replication?
Q. 138 What is incorrect about the following figure representing DNA replication?
A. the direction of DNA.replication in strand in (i)
B. the direction of DNA.replication in strand in (ii)
C. discontinuous replication of strand (i)
D. discontinuous replication of strand (ii)
![]() Q. 139 Which of the following is the characteristic of PS-I.
Q. 139 Which of the following is the characteristic of PS-I.
A. it is active only upto 680 nm of light
B. the reaction centre of PS-I is P680
C. PS-I is reduced by the electrons released in photolysis of water
D. PS-I is involved in non-cyclic photophosphorylation.
![]() Q. 140 Bark refers to
Q. 140 Bark refers to
A. phellem + phellogen + phelloderm
B. periderm + cortex
C. phellem + phelloderm + secondary phloem
D. periderm + cortex + pericycle + secondary phloem
![]() Q. 141 Cotton fibres mainly contains
Q. 141 Cotton fibres mainly contains
A. cellulose
B. glycogene
C. protein
D. lipids
![]() Q. 142 The outermost limiting layer of mycoplasma is made up of
Q. 142 The outermost limiting layer of mycoplasma is made up of
A. cell wall
B. cell membrane
C. mucilaginous sheath
D. slime layer
![]() Q. 143 Which of the following statements about Spirog’Ym is correct?
Q. 143 Which of the following statements about Spirog’Ym is correct?
A. ateral conjugation takes place in homothallic species
B. scalariform conjugation takes place in homothallic species
C. lateral conjugation takes place in heterothallic species
D. he type of conjugation is unrelated to homothallic & heterothallic species
![]() Q. 144 Which of the following sugars is not found in plants?
Q. 144 Which of the following sugars is not found in plants?
A. sucrose
B. glucose
C. lactose
D. fructose
![]() Q. 145 The binding site of tRNA with mRNA & amino acids respectively are
Q. 145 The binding site of tRNA with mRNA & amino acids respectively are
A. mRNA with DHU loop & amino acid with CCAend
B. m RNA with CCA end & amino acid with anticodon loop
C. mRNA with anticodon loop & amino acid with DHU loop
D. mRNA with anticodon loop & amino acid with CCA end
![]() Q. 146 Percentage of recombination between A and B is 9%, A and C is 17%, Band C is 26% , then the arrangement of genes is
Q. 146 Percentage of recombination between A and B is 9%, A and C is 17%, Band C is 26% , then the arrangement of genes is
A. ABC
B. ACB
C. BCA
D. BAC
![]() Q. 147 Which of the following is true?
Q. 147 Which of the following is true?
A. umbel is a racemose inflorescence where all stalked flower aggregate on the flat receptacle
B. raceme is a racemose inflorescence having main axis shortened & flower born acropetally
C. spadix is a racemose inflorescence having pendulous spike with main axis much flattened
D. spike is a racemose inflorescence having sessile flowers
![]() Q. 148 Jute fibres deteriorate quickly because
Q. 148 Jute fibres deteriorate quickly because
A. cellulose content is high
B. lignin content is high
C. cellulose content is low
D. lignin content is low
![]() Q. 149 The branched sclereids present in hydrophytes are
Q. 149 The branched sclereids present in hydrophytes are
A. ostcosclereids
B. trichosclereids
C. macrosclereids
D. astrosclereids
![]() Q. 150 The enzyme decarboxylase catalyses the following step
Q. 150 The enzyme decarboxylase catalyses the following step
A. conversion of citric acid to cis aconitic acid
B. fumaric acid to malic acid
C. oxalosuccinic acid to a-ketoglutaric acid
D. malic acid to oxaloacetic acid
![]() Q. 151 Which of the following is true regarding the given electron transport chain?
Q. 151 Which of the following is true regarding the given electron transport chain?
CoQ → Cyt c → Cyt aa₃ → O₂
A. CoQ → Cyt c is H⁺ absorbing site
B. aa₃ → O₂, H⁺ yielding site
C. CoQ → Cyt c is H⁺ yielding site and aa₃ → O₂ is H⁺ absorbing site
D. no H⁺ is absorbed or released
![]() Q. 152 Which one of the following is not a microelernent for plants?
Q. 152 Which one of the following is not a microelernent for plants?
A. Cu
B. B
C. Zn
D. Cr
![]() Q. 153 National bird of india is
Q. 153 National bird of india is
A. Psittacula
B. Passer domesiicus
C. Pauo cristatus
D. Parakeet
![]() Q. 154 Rain is called acid-rain when its pH is below
Q. 154 Rain is called acid-rain when its pH is below
A. 7 pH
B. 6.5 pH
C. 6 pH
D. 5.6 pH
![]() Q. 155 Cytokines that provide non specific immunity against virus are
Q. 155 Cytokines that provide non specific immunity against virus are
A. interleukin
B. tumour necrosi
C. colony stimulating
D. interferon
![]() Q. 156 By all of the following ways bacteria become resistant to antibiotic except
Q. 156 By all of the following ways bacteria become resistant to antibiotic except
A. making enzymes that inactivate the drug
B. becoming impermeable to the drug
C. modifying the target of the drug
D. moving away from the drug
![]() Q. 157 Specific proteins responsible for the flow of materials and information into the cell are called
Q. 157 Specific proteins responsible for the flow of materials and information into the cell are called
A. membrane receptors
B. carrier proteins
C. integral proteins
D. none of these
![]() Q. 158 Which of the following conditions represents a case of co-dominant genes?
Q. 158 Which of the following conditions represents a case of co-dominant genes?
A. a gene expresses itself f, suppressing the phenotypic effect of its alleles
B. genes that are similar in phenotypic effect. when present separately, but when
together interact to produce a different trait
C. alleles, both of which interact to produce a: trait, which mayor may not resemble either of the parental types
D. alleles, each of which produces an independent: effect in a heterozygous condition
![]() Q. 159 The first bioherbicide developed in 1981 was based on
Q. 159 The first bioherbicide developed in 1981 was based on
A. Phytopirthora palmiuora
B. Plrytoplrthorn in/estall
C. Bacilllls thuringiensis
D. Azadirachta indica
![]() Q. 160 Upon fertilization, what structure develops from carpel?
Q. 160 Upon fertilization, what structure develops from carpel?
A. Testa
B. tegman
C. pericarp
D. perisperm
![]() Questions: 161 – 180
Questions: 161 – 180
In the following questions (161-180), a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as :
(a) If both assertion and reason are true and reason is the correct explanation of assertion
(b) If both assertion and reason are true but reason is not the correct explanation of assertion
(c) If assertion is true but reason is false
(d) If both assertion and reason are false
![]() Q. 161 Assertion: Secondary succession takes place in • recently denuded area.
Q. 161 Assertion: Secondary succession takes place in • recently denuded area.
Reason : It is caused due to baring of an . area
A. A
B. B
C. C
D. D
![]() Q. 162 Assertion: Connective tissue inside the brain is essential for conduction of nerve impulse.
Q. 162 Assertion: Connective tissue inside the brain is essential for conduction of nerve impulse.
Reason : Connective tissue hold together the nerve cells of brain.
A. A
B. B
C. C
D. D
![]() Q. 163 Assertion: Mammary glands are apocrine glands.
Q. 163 Assertion: Mammary glands are apocrine glands.
Reason : The distal part containing secretory granules break down and leaves as a secretion.
A. A
B. B
C. C
D. D
![]() Q. 164 Assertion: Cytokinins increases shelf life of fruits and vegetables.
Q. 164 Assertion: Cytokinins increases shelf life of fruits and vegetables.
Reason : Cytokinins induce cell division.
A. A
B. B
C. C
D. D
![]() Q. 165 Assertion : Angina pectoris means “pain in the chest”.
Q. 165 Assertion : Angina pectoris means “pain in the chest”.
Reason : It results due to carrying of extra blood to the heart muscle.
A. A
B. B
C. C
D. D
![]() Q. 166 Assertion: Protandry and protogyny ensures cross fertilization.
Q. 166 Assertion: Protandry and protogyny ensures cross fertilization.
Reason : Cross fertilization introduces variation in progeny.
A. A
B. B
C. C
D. D
![]() Q. 167 Assertion: Bursa fabricii lies on the ventral side of the cloaca in birds.
Q. 167 Assertion: Bursa fabricii lies on the ventral side of the cloaca in birds.
Reason : Bursa fabricii is related with flight adaptation.
A. A
B. B
C. C
D. D
![]() Q. 168 Assertion: Glycolysis is the first step of respiration in which glucose completely breaks into CO2 and H20.
Q. 168 Assertion: Glycolysis is the first step of respiration in which glucose completely breaks into CO2 and H20.
Reason : In this process, there is net gain of twenty four molecules of ATP.
A. A
B. B
C. C
D. D
![]() Q. 169 Assertion: Restriction enzymes cut the strand of DNA to produce sticky ends.
Q. 169 Assertion: Restriction enzymes cut the strand of DNA to produce sticky ends.
Reason : Stickiness of the ends facilitates the action of the enzyme DNA polymerase.
A. A
B. B
C. C
D. D
![]() Q. 170 Assertion: Excess of nitrates in drinking water are harmful for infants.
Q. 170 Assertion: Excess of nitrates in drinking water are harmful for infants.
Reason : Nitrates are responsible for blue baby syndrome
A. A
B. B
C. C
D. D
![]() Q. 171 Assertion: Amniocentesis is a process of foetal sex determination.
Q. 171 Assertion: Amniocentesis is a process of foetal sex determination.
Reason : Metabolic errors and other diseases can be diagnosed prenatally by this process.
A. A
B. B
C. C
D. D
![]() Q. 172 Assertion: Pollen mother cells (PMCs) arc the first male gametophytic cells.
Q. 172 Assertion: Pollen mother cells (PMCs) arc the first male gametophytic cells.
Reason : Each PMC gives rise to two pollens.
A. A
B. B
C. C
D. D
![]() Q. 173 Assertion: Nucleus is the controlling centre of a cell.
Q. 173 Assertion: Nucleus is the controlling centre of a cell.
Reason : Pores in the nuclear envelop regulate the flow of materials in and out of the nucleus.
A. A
B. B
C. C
D. D
![]() Q. 174 Assertion: Hormone calcitonin has antagonistic effect to that of parathormone.
Q. 174 Assertion: Hormone calcitonin has antagonistic effect to that of parathormone.
Reason : Calcitonin decreases blood calcium level while parathorrnone increases blood calcium level.
A. A
B. B
C. C
D. D
![]() Q. 175 Assertion: Dark reaction occurs only at night in the stroma of chloroplast.
Q. 175 Assertion: Dark reaction occurs only at night in the stroma of chloroplast.
Reason : CO2 fixation occurs only during C3 cycle.
A. A
B. B
C. C
D. D
![]() Q. 176 Assertion: The primitive atmosphere was reducing one i.e. without oxygen.
Q. 176 Assertion: The primitive atmosphere was reducing one i.e. without oxygen.
Reason : In the primitive atmosphere, oxygen was involved in forming ozone layer
A. A
B. B
C. C
D. D
![]() Q. 177 Assertion: Jave Ape-man, Peking man and Heidelberg man are the fossils of Homo crcctus.
Q. 177 Assertion: Jave Ape-man, Peking man and Heidelberg man are the fossils of Homo crcctus.
Reason : Homo erecius evolved from Homo hnbilie.
A. A
B. B
C. C
D. D
![]() Q. 178 Assertion: Loss of water produces a negative hydrostatic pressure.
Q. 178 Assertion: Loss of water produces a negative hydrostatic pressure.
Reason : Positive hydrostatic pressure is developed due to osmotic entry of water in.o it.
A. A
B. B
C. C
D. D
![]() Q. 179 Assertion:Mammalian ova produces hyaluronidase.
Q. 179 Assertion:Mammalian ova produces hyaluronidase.
Reason : TIle eggs of mammal are microlecithal and telolecithal.
A. A
B. B
C. C
D. D
![]() Q. 180 Assertion: A gamete may carry either of the traits but not both.
Q. 180 Assertion: A gamete may carry either of the traits but not both.
Reason : This is Mendel’s second law or law of independent assortment.
A. A
B. B
C. C
D. D
![]() Q. 181 A pendulum clock is set to give correct time at the sea level. The clock is moved to a hill station at an altitude 11 above sea level. In order to keep correct time on the hill station which one of the following adjustments is required?
Q. 181 A pendulum clock is set to give correct time at the sea level. The clock is moved to a hill station at an altitude 11 above sea level. In order to keep correct time on the hill station which one of the following adjustments is required?
A. the length of the pendulum has to be reduced
B. the length of the pendulum has to be increased
C. the mass of the pendulum has to be increased
D. the mass of the pendulum has to be reduced
![]() Q. 182 Match List I with List II (Given in the figure) and select the correct answer using the codes given below:
Q. 182 Match List I with List II (Given in the figure) and select the correct answer using the codes given below:
A. A(4), B(1), C(2), D(3)
B. A(3), B(1), C(2), D(4)
C. A(3), B(2), C(1), D(4)
D. A(4), B(2) C(1) D(3)
![]() Q. 183 The President of India is elected by
Q. 183 The President of India is elected by
A. members of both Houses of Parliament
B. members of both Houses of Parliament and of State Legislatures
C. members of both Houses of Parliament and of State Legislative Assemblies
D. elected members of both Houses of Parliament and elected members of state Legislative Assemblies
![]() Q. 184 Who wrote the book ‘India Wins Freedom’?
Q. 184 Who wrote the book ‘India Wins Freedom’?
A. Maulana Abul Kalam Azad
B. Mahatma Gandhi
C. Sir Mohammad Iqbal
D. Abdul Ghaffar Khan.
![]() Q. 185 Consider the following statements about the National Anthem:
Q. 185 Consider the following statements about the National Anthem:
It was first sung on December 27, 1911 at the Calcutta session of the Indian National Congress. It was adopted by the Constituent Assembly on January 26, 195 Playing time of the full version of the National Anthem is approximately 52 second Which of the statements given above is/are correct?
A. 1 only
B. 1 and 2
C. 1 and 3
D. 2 and 3
![]() Q. 186 Match List I with List II (Given in the figure) and select the correct answer using the codes given below:
Q. 186 Match List I with List II (Given in the figure) and select the correct answer using the codes given below:
A. A(2), B(1), C(5), D(4)
B. A(3), B(4), C(2), D(5)
C. A(3), B(2), C(4), D(1)
D. A(2), B(1), C(3), D(5)
![]() Q. 187 The metal compound commonly found in Sindhoor or Kumkum is based on
Q. 187 The metal compound commonly found in Sindhoor or Kumkum is based on
A. tin
B. lead
C. copper
D. zinc
![]() Q. 188 Which among the following thermometers is. preferred for measuring temperature around 1250°C?
Q. 188 Which among the following thermometers is. preferred for measuring temperature around 1250°C?
A. mercury thermometer
B. mercury thermometer
C. optical pyrometer
D. platinum resistance thermometer
![]() Q. 189 The term stagflation refers to a situation where
Q. 189 The term stagflation refers to a situation where
A. growth has no relation with the change in prices
B. rate of growth and prices both are decreasing
C. rate of growth is faster than the rate of price increase
D. rate of growth is slower than the rate of price increase
![]() Q. 190 Which event brought about a profound change in Ashoka’s administrative policy?
Q. 190 Which event brought about a profound change in Ashoka’s administrative policy?
A. the third Buddhist council
B. the Kalinga war
C. his embracing of buddhism
D. his sending of missionary to Ceylon.
![]() Q. 191 The plant dye Henna imparts orange-red colour to skin and hair due to its reaction with which of the following?
Q. 191 The plant dye Henna imparts orange-red colour to skin and hair due to its reaction with which of the following?
A. proteins and amino acids
B. lipids
C. carbohydrates
D. nucleic adds.
![]() Q. 192 Match List I with List II (Given in the figure) and select the correct answer using the codes given below:
Q. 192 Match List I with List II (Given in the figure) and select the correct answer using the codes given below:
A. A(2), B(3), C(4), D(1)
B. A(1), B(4), C(3), D(2)
C. A(2), B(4), C(3), D(1)
D. A(1), B(3), C(4), D(2)
![]() Q. 193 Delingha came recently in news? What is this?
Q. 193 Delingha came recently in news? What is this?
A. it is an endangered species of an Indian bird
B. it is place in Europe which was struck by an earthquake
C. China recently deployed missiles in the Delingha near Tibet sending alarming signals across political establishment in India
D. None of these
![]() Q. 194 Octopus is an anti-terror ageny of
Q. 194 Octopus is an anti-terror ageny of
A. Kerela
B. Andhra Pradesh
C. Karnataka
D. Gujarat
![]() Q. 195 Indira Gandhi Prize for peace, disarmament and development has recently been conferred on
Q. 195 Indira Gandhi Prize for peace, disarmament and development has recently been conferred on
A. Bill gates
B. Jacob Zurna
C. Asma Jahangir
D. None of the above
![]() Q. 196 India’s Deep Joshi has recently been honoured with
Q. 196 India’s Deep Joshi has recently been honoured with
A. Magsaysay Award
B. Whitely Prize
C. Right to Livelihood Award
D. None of these
![]() Q. 197 The runner up in 2009Wimbledon Men’s Singles was
Q. 197 The runner up in 2009Wimbledon Men’s Singles was
A. Roger Federer
B. Rafael Nadal
C. Andy Roddick
D. None of the above
![]() Q. 198 Who amongst the following cricketers has been chosen for Rajiv Gandhi Khel Ratna Award, 2007?
Q. 198 Who amongst the following cricketers has been chosen for Rajiv Gandhi Khel Ratna Award, 2007?
A. Rahul DravID
B. M.S Dhoni
C. Sachin Tendulkar
D. Virender Sehwag.
![]() Q. 199 Who amongst the following became the first woman pilot in the world to fly MIG-35 fighter plane?
Q. 199 Who amongst the following became the first woman pilot in the world to fly MIG-35 fighter plane?
A. Suman Sharma
B. Saudamini Deshmukh
C. Kirsty Moore
D. Nicole Malachowski.
![]() Q. 200 Mr. Paul Krugman whose name was in news recently is a famous
Q. 200 Mr. Paul Krugman whose name was in news recently is a famous
A. medical scientist
B. econimist
C. author
D. astrophysicist.
| Question | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Answer | A | A | C | C | B | C | C | A | A | A |
| Question | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Answer | D | C | C | D | A | B | D | A | A | B |
| Question | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Answer | A | B | A | B | A | B | C | B | C | D |
| Question | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 |
| Answer | D | A | B | A | C | A | D | C | A | C |
| Question | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 |
| Answer | C | B | C | D | C | A | A | A | A | B |
| Question | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 |
| Answer | B | C | A | B | C | A | A | A | A | C |
| Question | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 |
| Answer | C | B | D | C | C | A | B | D | D | D |
| Question | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 |
| Answer | A | B | B | B | C | C | A | B | B | C |
| Question | 81 | 82 | 83 | 84 | 85 | 86 | 87 | 88 | 89 | 90 |
| Answer | A | A | A | C | B | C | A | C | D | B |
| Question | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 100 |
| Answer | D | A | B | A | A | C | C | A | B | A |
| Question | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | 109 | 110 |
| Answer | A | C | A | C | A | A | B | C | A | B |
| Question | 111 | 112 | 113 | 114 | 115 | 116 | 117 | 118 | 119 | 120 |
| Answer | C | A | B | D | B | C | C | D | B | A |
| Question | 121 | 122 | 123 | 124 | 125 | 126 | 127 | 128 | 129 | 130 |
| Answer | D | B | B | B | A | D | C | B | C | A |
| Question | 131 | 132 | 133 | 134 | 135 | 136 | 137 | 138 | 139 | 140 |
| Answer | B | D | B | A | B | C | B | C | D | D |
| Question | 141 | 142 | 143 | 144 | 145 | 146 | 147 | 148 | 149 | 150 |
| Answer | A | B | A | C | D | D | D | B | D | C |
| Question | 151 | 152 | 153 | 154 | 155 | 156 | 157 | 158 | 159 | 160 |
| Answer | C | D | C | D | D | D | B | D | A | C |
| Question | 161 | 162 | 163 | 164 | 165 | 166 | 167 | 168 | 169 | 170 |
| Answer | A | D | A | B | C | B | D | D | C | A |
| Question | 171 | 172 | 173 | 174 | 175 | 176 | 177 | 178 | 179 | 180 |
| Answer | B | D | B | A | D | C | B | D | D | C |
| Question | 181 | 182 | 183 | 184 | 185 | 186 | 187 | 188 | 189 | 190 |
| Answer | A | B | D | A | C | B | D | D | D | B |
| Question | 191 | 192 | 193 | 194 | 195 | 196 | 197 | 198 | 199 | 200 |
| Answer | A | A | C | B | A | A | C | B | A | B |